The availability of oxazine leucos is dictated by the ease of synthesis of the oxazine dyes.
The purple phenoxazone 36 is obtained by alkaline hydrolysis of Basic Blue 3.14 The benzophenoxazone 38 is obtained by coupling of 2-nitroso-5- diethylaminophenol with a-naphthol.14 The leucos 37 and 34 are obtained by refluxing the corresponding dyes with zinc powder in acid anhydride.
|
|
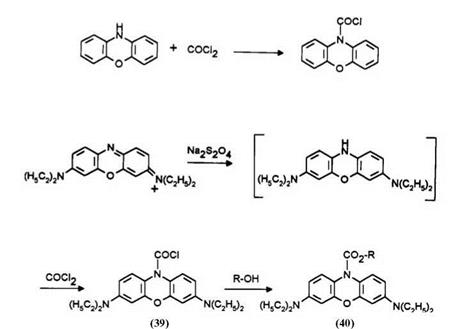
Based on the well-known reaction of phenoxazine with phosgene,15 leuco Basic Blue 3 was treated with phosgene to form the stable 10- chlorocarbonyl-3,7-diethylaminophenoxazine (39) which can be isolated and further reacted with alcohol or amine.16
|
|
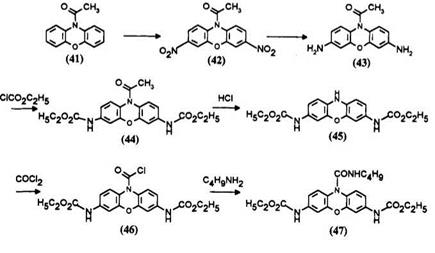
Starting from phenoxazine, the leuco 47 is obtained through a multistep reaction involving nitration, reduction, and acylation.6
|
|
Synthetic Method 6: 9-diethylamino-5-(N-acetyl-N-benzylamino)-12-
acetylbenzo[a ]phenoxazine (33) (procedure from U. S. Patent 4,604,461). 12 A mixture of 14.0 g of 9-diethylamino-5-(N-benzylamino)benzophenoxazin — 7-ium chloride, 75.0 ml of acetic anhydride, 5.0 ml of pyridine, and 7.0g of zinc dust was maintained at reflux temperature for approximately 2 h. After cooling to room temperature, the reaction mixture was filtered to remove the insoluble material and the filter cake was washed twice, each time with 50 ml of acetone. The filtrate and the washes were combined, concentrated, and slowly poured into a stirred mixture of water and toluene. After stirring the mixture for 30 min, the organic layer was separated, treated with decolorizing charcoal, filtered, and the resulting clarified toluene solution was evaporated under reduced pressure to afford 15.17g of 9-diethylamino — 5(N-acetyl-N-benzylamino)-12-acetylbenzo[a]phenoxazine as a pale brown powder which melted over the range of 86 to 91°C.
Synthetic Method 7 : 1,9-dimethyl-4-acetarnido-8-diethylaminoimidazo — phenoxazine (31) (procedure from U. S. Patent 4,604,462). 12 A mixture of 10 g of 1,3-diamino-7-diethylamino-8-methylphenoxazin-5-ium chloride (Brilliant Cresyl Blue), 50 ml of acetic anhydride, 10 g of zinc dust, and 10 ml of pyridine was maintained at 85 to 90°C for approximately 1 h. After cooling to room temperature, the reaction mixture was poured into a mixture of water and toluene and the resulting aqueous layer was discarded. The toluene solution was washed twice, first with water and then with
saturated sodium chloride solution. The toluene was removed by evaporation at reduced pressure. The residue was recrystallized from isopropanol and the solid obtained was recrystallized from ethanol to obtain 0.2g of 1,9-dimethyl-4-acetamido-8-diethylaminoimidazophenoxazine as a white solid which melted at 217 to 218°C.
 21 июля, 2015
21 июля, 2015  Malyar
Malyar 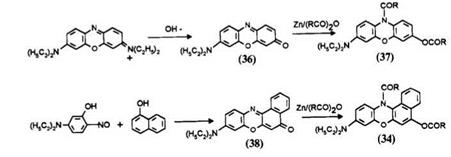
 Опубликовано в рубрике
Опубликовано в рубрике 