The thiazine dyes used in the preparation of this type of leuco are obtained through oxidative coupling of phenothiazine with an active methylene compound or an aniline. The reduction of the dye 23 with zinc powder in acetic acid is straightforward.9 Treatment of the leuco 24 with acetic anhydride at 40°C yields a more air stable leuco 25.9 Addition of arylsulfinic acid to thiazine dyes such as 26 produces directly leuco dyes such as 27.8b
|
|
Synthetic Method 4: 3-(4-carbethoxyanilino) — phenothiazine (13n) (procedure from U. S. Patent 4,710,570).Sb To a warm and stirred suspension of 4.0g (0.02 mol) of finely powdered phenothian zine and 4.0g (0.024 mol) of ethyl-p-aminobenzoate in 200ml of methanol was added a solution of 10.0 g of iodine in 150ml of methanol. After stirring at room temperature for 2 h, the dark precipitate was filtered, washed repeatedly with methanol, and dissolved in l00ml of chloroform and 10ml of triethy- lamine. The chloroform solution was shaken with water and separated. The aqueous layer was discarded and the organic layer evaporated. The residue was purified through alumina. Recrystallization from ether gave 5.5 g (76%) of the dye 3-(4-carbethoxyphenylimino)-3#-phenothiazine as purple leaflets.
To a stirred suspension of 1.0g of the above dye in some 50ml of warm acetone was added excess zinc dust and a few drops of concentrated hydrochloric acid. The mixture was stirred until the coloration discharged, and then filtered. The cake was extracted with hot acetone. The filtrate and extracts were combined, concentrated, and poured into water. The precipitate was filtered, washed repeatedly with water and cold methanol, and dried. Recrystallization from methanol gave 0.9g (90%) of the leuco dye 3-(4-carbethoxyanilino)phenothiazine as a white powder which gradually turned pinkish on contact with air.
Synthetic Method 5: 2-benzenesulfonyl-3-(p-carbethoxyanilino)pheno-
thiazine (13o) (procedure from U. S. Patent 4,710,570).8b To a warm, stirred solution of 1.8 g of dye 3-(4-carbethoxyphenylimino)-3H-pheno — thiazine in 50 ml of tetrahydrofuran was added 0.8 g of benzenesulfinic acid (obtained by adding dilute hydrochloric acid to an aqueous solution of benzenesulfinic acid sodium salt to cause precipitation of the free acid). After stirring 1 h at 40°C the solvent was evaporated off under reduced pressure. The yellowish solution was poured into water. The precipitate was filtered, washed repeatedly with distilled water and methanol. Recrystallization from methanol gave 1.9 g (76%) of the leuco dye 2-benzenesulfonyl-3-(4-car — bethoxyanilino)phenothiazine (13o) as a yellowish powder.
 16 июля, 2015
16 июля, 2015  Malyar
Malyar 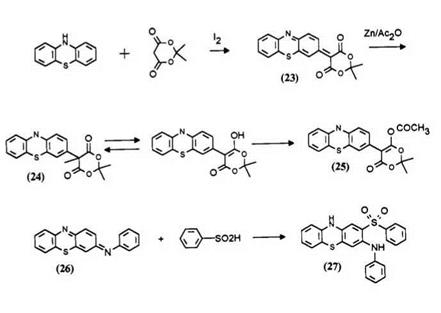
 Опубликовано в рубрике
Опубликовано в рубрике 