Methylene Violet, which is a well-known phenothiazinone dye, is also reduced and acylated in one step by refluxing with zinc powder in an acid anhydride.
|
|
Synthetic Method 3: 6-dimethylamino-3-acetoxy-10-acetylphenothiazine (11a) (procedure from US. Patent 4,604,458)? Under a nitrogen atmosphere, a mixture of 5.0 g of 7-dimethylaminophenothiazin-3-one (Methylene Violet), 75.0ml of acetic anhydride, 5.0ml of pyridine, and 50g of zinc dust was maintained at reflux temperature for approximately 3 h. After cooling to room temperature, the reaction mixture was filtered to remove the insoluble materials and the filter cake was washed twice, each time with 50.0ml of acetone. The combined filtrate and acetone washes were concentrated and poured slowly into water and 100ml of toluene was added to the resulting mixture. After stirring for approximately 30 min, the layers were separated and the aqueous layer was discarded. The organic extract was treated with activated charcoal, filtered, and the toluene was evaporated off at reduced pressure to obtain a gummy residue. The residue was dissolved in ethylacetate and the resulting solution was passed through a chromatographic column packed with silica gel. Elution with ethylacetate yielded a solid as a white powder (2.6g) which melted at 124 to 128°C.
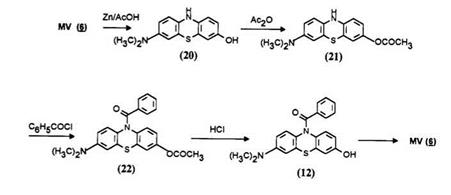
Reduction of Methylene Violet with zinc in acetic acid gives the air — sensitive leuco 20 which is further reacted with acetic anhydride in mild conditions to yield the acetylated leuco 21. The latter being air stable can be isolated and, the ring N-H being less reactive is not affected by acetylation at room temperature. The leuco 21 is again aroylated to produce the leuco 22. Selective hydrolysis provides the desired leuco dye 12 which regenerates the true Methylene Violet (6) on oxidation.8a
|
|
 16 июля, 2015
16 июля, 2015  Malyar
Malyar 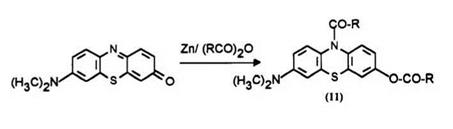
 Опубликовано в рубрике
Опубликовано в рубрике 