These leuco dyes are stable enough to be isolated without the need for acylation. Some are more resistant than others to air oxidation depending on their redox potential.9
|
|
As shown in Table 1, the introduction of an electron-withdrawing group raises the redox potential and stabilizes the leuco against air oxidation. In one extreme case, this stabilization has become so efficient that leuco 13o is too stable to be oxidized back to the dye, thus severely limiting its usefulness as an imaging material.
Table 1. Effect of Substituents on the Redox Potential and Reactivity
of Thiazine Leuco Dyes
|
(13) |
|
R1 |
R2 |
Eox |
|
|
a |
CH(CO—C6H5>2 |
H |
— |
|
b |
CH(CN)2 |
H |
— |
|
c |
CH(CN)2 |
SO2C6H5 |
+ 0.70 V |
|
d |
CH(CN)CO2 c2h5 |
H |
— |
|
e |
CH(CN)CO2 c2h5 |
SO2C6H5 |
+ 0.65 V |
|
f |
CH(CN)CONH2 |
H |
+ 0.54V |
|
g |
CH(CN)CONH22 |
SO2C6H5 |
+ 0.69V |
|
h |
CH(CN)CO—C6H5 |
H |
— |
|
i |
H |
+ 0.25V |
|
|
j |
-H-® |
SO2C6H5 |
+ 0.29V |
|
k |
CH3 |
SO2C6H5 |
+0.31V |
|
1 |
-й-Q-a |
H |
+0.25V |
|
m |
-Я-О-сі |
SO2C6H5 |
+0.35V |
|
n |
H |
+0.35V |
|
|
o |
-N-Q-C°2C2H5 |
SO2C6H5 |
> + 1.0V |
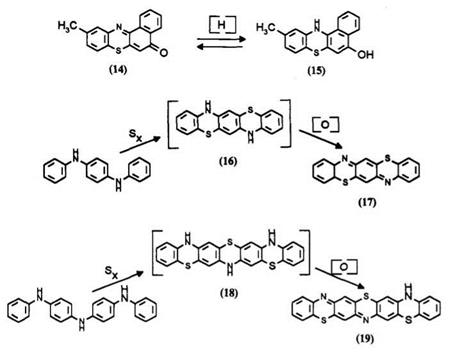
Benzo[1,2-a]-8-methyl-9-azaphenothiazinone (14) was reduced to a leuco form 15 which was too unstable to be isolable.10 The leucos 16 and 18 obtained from thionation of N^-diphenyl-p-phenylenediamine and j9,p’-dianilinodiphenylamine, respectively, are also air sensitive. 11 They are oxidized to thiazine dyes 17 and 19 which are reported to absorb in the near infrared.
 |
 15 июля, 2015
15 июля, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 