When Methylene Blue is reduced, the yellowish leuco cannot be isolated due to instant air oxidation. Benzoylation of the leuco form provides stabilization. There are also leuco thiazine dyes stable enough to be isolated without the need for aroylation.
3.2.1. Acylated Leuco Thiazine Dyes
 |
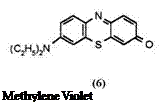 |
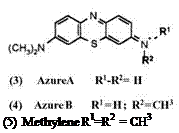
There are cationic thiazine dyes (3 to 5) and neutral thiazinone dyes exemplified by Methylene Violet (6). Like leuco Methylene Blue, leuco Methylene Violet is too air sensitive to be isolated and therefore requires acylation.
3.2.1.1. AcylatedLeuco Cationic Thiazine Dyes
The leucos 7a-d are described as useful in printing ink for preventing forgery,1 whereas 7e-g are used in pressure sensitive copying paper.2
The leucos 7h-o are claimed in electrolytic recording paper using a process coined “electrochromic recording” which is an irreversible electrooxidation of the leuco dye to regenerate Methylene Blue, not to be confused with reversible electrochromic display. The process consists of passing an electrical pulse through a substrate containing the leuco dye and
ammonium bromide to generate bromine in-situ which acts as an oxidizing agent for leuco Methylene Blue.3 The leuco 7p is claimed as a sublimable leuco dye in thermal dye transfer imaging wherein it is converted to Methylene Blue on the receptor sheet by an incorporated oxidizer.4
![]() “► Br5
“► Br5
 |
Br2 + LEUCO
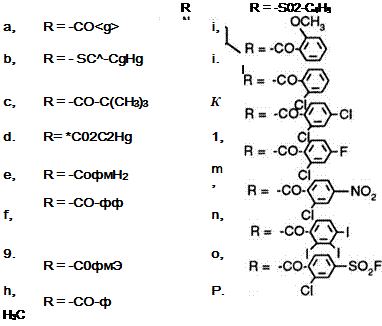
Instead of using Methylene Blue, Azure B is reduced and acylated.4 The resulting leucos 8a-t are also described as useful in electrolytic recording.5
(8) ‘
|
a. |
R= CH3 |
k. |
R=-O"0CH3 |
|
b, |
R = CHjCCHjJj |
i, |
R= |
|
c, |
R = — |
m. |
R=~P H3C |
|
d, |
R= CH2-0-CH3 |
n, |
R = Br |
|
e, |
R= — O |
ot |
».-p |
|
f. |
R= с5ни |
P, |
CFa R—P-F p |
|
9. |
R = CCI3 |
q. |
R= -<2>-C02H |
|
h, |
R= CH2-0-O |
rt |
R = -©-N02 |
|
i, |
R= |
st |
r=-£)-CN |
|
ji |
R= — Q-CH3 |
t, |
In contrast to leuco Methylene Blue, acylated leuco Azure B fails to regenerate the original dye by virtue of the fact that the exocyclic amino group was also acylated during the leuco dye synthesis and the amide group remains on oxidation. Acylated Azure B is formed instead resulting in a different color. The leucos 8a-t are reported to give green black or blue black images.
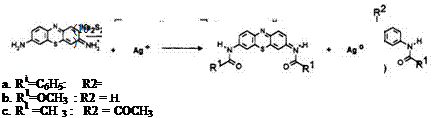 |
Safranine O is reduced to a leuco intermediate and acylated in one step to produce the leuco 9. Only the exocyclic amino groups are acylated at this point due to their higher reactivity. The ring amino groups can then be acylated with another acid chloride to produce the safranine leucos 10a-c which are claimed to give pink or purple images in Color Dry Silver. In this application, light-sensitive silver halide is the oxidizing species.6
 13 июля, 2015
13 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 