In the past, various leuco benzoquinone dyes4 were used as mordant dyes but recently they have been displaced by the azo mordant dyes. The reaction of p-benzoquinone with p-chloroaniline gives the hydroquinone derivative (5). Compound 5 undergoes oxidation to the corresponding benzoquinone 6. A mixture of hydrosulfite and compound 6 is marketed as a sulfurized vat dye which gives brown and khaki colors.
|
|
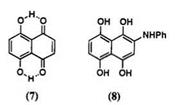
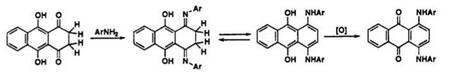
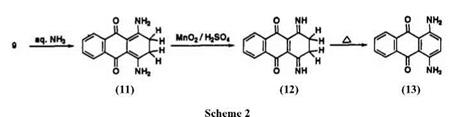
In the naphthoquinone series, naphthazarin (7)5 had considerable commercial importance at one time. Reduction of 7 gives 1,4,5,8-tetrahy — droxynaphthalene (naphthazarin leuco form) which has been used to dye wool and silk from the leuco form. After oxidation of the leuco form with a metal ion such as chromium, a chelated dye is obtained giving a neutral black with good all-around fastness. Naphthazarin readily reacts with amines and phenols. Many mordant dyes such as Alizarin Black SRA (8) have been synthesized from naphthazarin. While these naphthoquinone dyes are of little technical importance, the leuco quinizarin (9) is an important intermediate for the synthesis of 1,4-disubstituted anthraquinone dyes. The reaction of 9 with arylamine followed by oxidation gives 1,4-bis(aryl — amino)anthraquinones (10) (Scheme 1).4 Interestingly, when 9 is heated with aqueous ammonia, only 1,4-diamino-2,3-dihydroanthraquinone (11) is formed. Oxidation of 11 with manganese dioxide leads to 12, which on heating at about 100°C isomerizes to 1,4-diaminoanthraquinone (13) (Scheme 2).4
|
|
|
|
|
|
 6 июля, 2015
6 июля, 2015  Malyar
Malyar 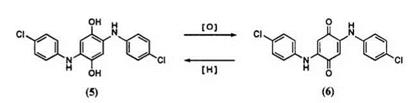
 Опубликовано в рубрике
Опубликовано в рубрике 