The absorption band of the colored form of spironaphthooxazine has been measured in thermal equilibrium with the spiro form, particularly in a polar solvent at low temperature. Typical absorption spectra of the colorless and the colored form in polymer films are shown in Figure 1.8.
|
Figure 1.8. Absorption spectra of (a) the colorless form and (b) the colored form of 1-buty1-3,3-dimethy1-spiroindolinonaphthooxazine produced by irradiation with UV light in PVC film (1.0 wt%) at 23°C. |
The thermal fading rate of the colored form can be stopped at low temperature (- 60 to — 75°C). For example, at — 75°C the lmax and molar absorptivity of the colored form of spironaphthooxazine 33 have been found experimentally to be 612 (є = 8 * 104 dm-3 M-1 cm-1) and 578nm (є = 4.9 * 104 dm -3 M -1cm-1).77
In contrast to normal spiropyrans, in spironaphthooxazine series, as the polarity of the solvent decreases, a hypsochromic shift of the Xmax of the colored form is observed, except for spiropiperidinonaphthooxazine.79 For example, the Xmax of 33 shifts to shorter wavelength of ca. 20-60 nm in less polar solvents, such as toluene and cyclohexane, compared to ethanol. This result may suggest that the ground state of the photomerocyanine form in spironaphthooxazine is less polar than the excited state and the neutral quinoid form largely contributes to the photomerocyanine form in the ground state.
The Хщи of 33 shows a bathochromic shift, compared to that of the corresponding spironaphthopyran [Xmax 531, 558(s) nm in toluene].78 The substituent effect in 2′,5′,6′- and 5-position of 33 on the absorption band of the colored form has been examined.72,77,78 The donor substituent group in 6′-position, such as piperidino group, gives a hypsochromic shift by 35 nm, but 5′-carbomethoxy substitution results in a bathochromic shift by 20 nm. This may be due to interaction between oxygen atom of the phenolate and methoxy group.
Unlike spiropyran, 2′-substitution in spirooxazine has no effect on X max Alkoxy, chloro, and nitro substituents at 5-position, and alkyl substituent at 1-position in the indoline component have small effects on the Xmai of the colored form.
Extension of п-conjugation from naphthalene to anthracene and phenanthrene has a small effect on the X max of the photomerocyanine form. Replacement of the indoline ring with piperidine, benzoxazole, or benzo- thiazole83 has resulted in hypsochromic shift by ca. 10 nm.72
 27 июня, 2015
27 июня, 2015  Malyar
Malyar 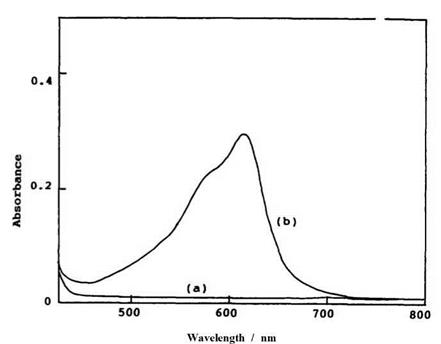
 Опубликовано в рубрике
Опубликовано в рубрике 