Many 2,2-dialkylchromenes, e. g., gambogic acid and flemingins, have been discovered in nature.48 2,2-Dialkylchromenes exhibit photochromic behavior, and their colored forms generally show yellow to orange hue.57 The chromene structure is thermally stable at room temperature, and the colored form is produced by irradiation in approximately 50s in toluene at 24°C.58 Studies on the mechanism of photocoloration of 2,2′-dialkyl — chromene indicate that the colored form for chromenes containing no nitro group occurs from the excited singlet state of the chromene59 and for nitro-substituted chromenes, it occurs partly from the excited singlet state and partly from the triplet state of the cisoid form.
If R or R’ is not an amino group, the ortho-quinoid structure 24b is preferred over the zwitterionic structure 24c for the colored form, since there is no additional conjugation to stabilize the carbonium ion produced by irradiation. Kolc and Becker first postulated the ortho-quinoid structure for the colored form which was produced at low temperature and trapped by reduction with LiAlH4.60 It is reported that the cisoid form produced by photoirradiation undergoes a 1,7-hydrogen shift to the phenol 25 when R is a methyl group (Scheme 13).61 When R (or R’) is a donor group, such as an amino group, the zwitterionic form is stabilized, and the Xmax of the colored form is significantly redshifted. For example, the colored form of 6-nitro-8-methoxy-2,2,3-trimethylchromene has two bands at 370 and 500m.58 In the case of 2-(A-carbazolyl)-6-nitro chromene (24, R’ = H, R = Wcarbazolyl), the X max is at 520 nm. The colored form of 2-methyl-2-(p — diethylaminopheny1)naphthopyran 26 also absorbs at 550 nm.62
Merlini has presented a detailed review of preparation of some 2,2-di — alkylchromenes.48
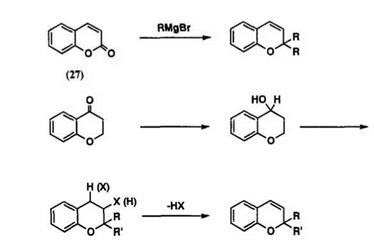
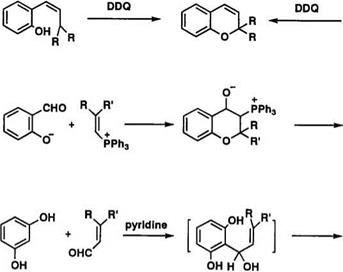
The 2,2-dialkylchromenes can easily be obtained from the reaction of coumarin 27 with a Grignard reagent.48 This method has been known for a long time and has not been modified much. The parent chromene 28 has been prepared by reduction and dehydration of 4-chromanone.63 Elimin-
|
ation of HX from 2,2-dialky1-3- or 4-halochroman 29 by base also gives
2.2- dialky1hromenes64 (Scheme 14).
Oxidation of оф-unsaturated (or p, y-unsaturated) o-propenylphenol or
2.2- dialky 1chroman with 2,3-dichloro-5,6-dicyano- 1,4-benzoquinone (DDQ) gives chromene derivatives.65,66 2,2-Dialky 1chromene is also obtained by
 n
n
H
|
|
the Wittig reaction from salicylaldehyde and vinylphosphonium salt,48,67 or by condensation of оф-unsaturated aldehydes with resorcinals in the presence of pyridine 68,69 (Scheme 15).
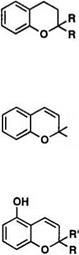
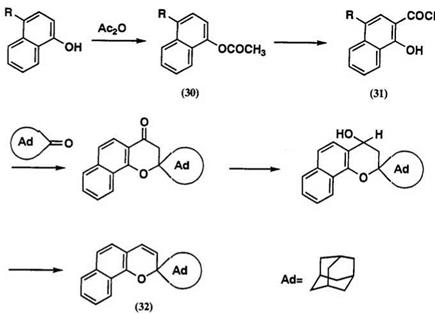
The colored form of spironaphthopyran 32 absorbs at ^max of ca. 450 m,70 and the closed spiro form is colorless, which has no absorption band above 400nm. Bulky substituent group is especially important for photochromic sunglass. Introduction of the spiroadamantane or spirobi — cyclo [3.3.1]heptane into the 2-position of naphthopyran increases the resistance to photo-fatigue reaction, since endocyclic double bond induced by 1,7-hydrogen shift in the colored form cannot be formed in 2-adamanty1 or 2-bicycloheptany1 group.
Typical preparation of naphthopyran 32 involves Fries rearrangement of 1-acetoxynaphthalene 30. Condensation of 2-acetyl-1-naphthol 31 with adamantanone, followed by usual reduction and dehydration gives 32 (Scheme 16).70
The colored form of 2,2-diphenyl-2#-thiochromene (thio analogue of chromene) absorbs at 650 nm in 3-methylpentane at 77 K.71
 26 июня, 2015
26 июня, 2015  Malyar
Malyar 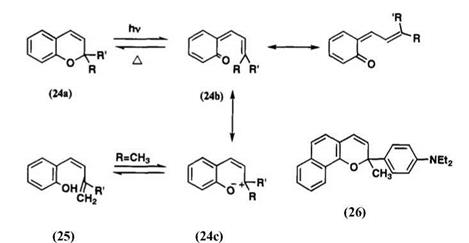
 Опубликовано в рубрике
Опубликовано в рубрике 