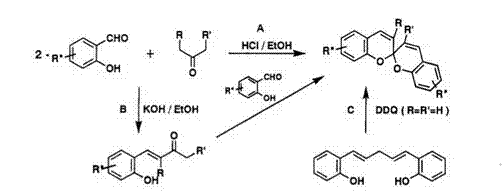
Spirodibenzopyrans are prepared by two principal routes: (A) condensation of two equivalents of salicylaldehyde with appropriate ketone; (B) condensation of o-hydroxystyryl ketone 15, which is prepared from salicylaldehyde and a ketone in the presence of KOH, with the same or different salicylaldehyde derivatives, as shown in Scheme 10.2,48 Spirodibenzopyran can also be obtained from 1,5-bis(2-hydroxypheny1)-1,4-pentadiene 16 by dehydrogenative reaction (C).48
Method B involves the preparation of precursor of 2-alky1-1-benzo — pyrylium salts, as shown in Scheme 1 1.50 2-Alky1benzopyrylium salts have been prepared by condensation of salicylaldehyde with appropriate ketone in acetic acid or by alkylation or reduction of coumarin or chromone derivatives. Reaction of 2-alky1benzopyrylium salts with salicylaldehyde gives directly a spirodibenzopyran or 2-vinynologue benzopyrylium salt 17 which then can be converted into the spirodibenzopyran by piperidine or pyridine.
|
Scheme 11 |
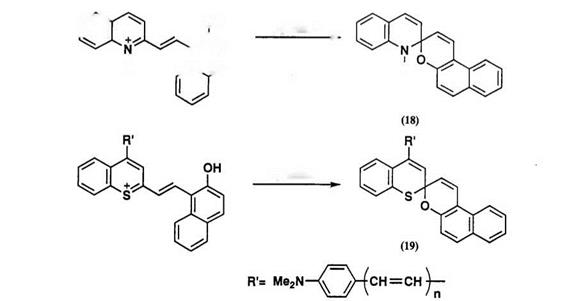
Heteroanalogues of spirodibenzopyran, such as spiroquinolinonaph — thopyran 18 and spirobenzothiopyranonaphthopyran 19, are similarly prepared from the corresponding quinolinium salts and benzothiopyrylium salts, respectively (Scheme 12)51’52 Spirobenzothiopyranonaphthopyran 19 has an extended л-conjugation in 4′-position, and its photomerocyanine
|
n |
OH |
pyridine |
||
|
тґЧ |
* |
|||
|
1 R ^ |
Ij |
R |
 n=0~2
n=0~2
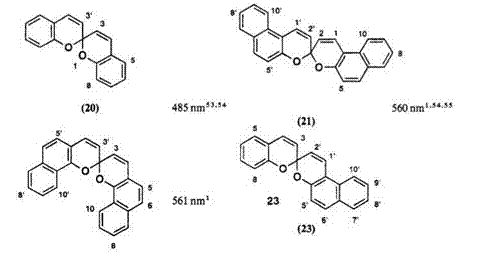
Table 4. Xmax of the Photocolored Form of the Spirodibenzopyran Series in
 |
1,4-Dioxane
form absorbs in the near infrared region (692-858nm). However, this photomerocyanine form is stable and is not reversed to the spiropyran form.52
The colored form of spirodibenzopyran can also be regarded as merocyanine chromophore. For photomerocyanine structure, the contribution of the zwitterionic form (14b) may be greater than the quinoid form (14c), since the stability of the charge separation leads to favorable formation of the aromatic benzopyrylium ring and benzene ring. However, the Xmax of colored forms in some spirodibenzopyrans shifts to longer wavelength when polarity of the solvent is increased,1 suggesting that the quinoid form is dominant in the photocolored form.
Annelation of the benzopyran in spirodibenzopyran series results in remarkably bathochromic shift of the absorption band in the colored form, as shown in Table 4. The Xmax of the colored form in 21-23 shifts to longer wavelength of about 80-100 nm, compared to the parent spirodibenzopyran 20.
Substituent effects on the Xmax of the colored form in these series are interesting and are dependent on the pyran component. For compound 20, substitutions at position 3, 6, or 8 (or 3′, 6′, 8′) affects the Xmax substantially, i. e., the absorption band lies in a wide range 475-609nm. The 7,7′-, 8,8′-, or 10,10′-dinitro-substituted compounds 21 reveal a somewhat hypso — chromic shift, relative to unsubstituted derivative, whereas the bathochromic shift (10-40 nm) is observed with dinitro substitution at the same position in compound 22. For compound 23, the pronounced shift is also observed with 8-nitro substitution. Many other spectral data are collected in Ref. 1.
 25 июня, 2015
25 июня, 2015  Malyar
Malyar 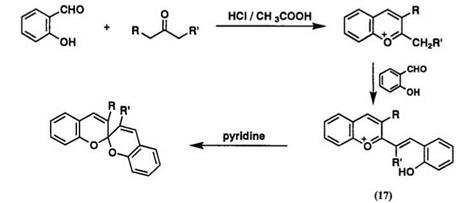
 Опубликовано в рубрике
Опубликовано в рубрике 