Generally, measurement of absorption spectra of the colored form of spirobenzopyran is very difficult using normal spectrophotometry, as the colored form is thermally unstable. The absorption spectra of the colored form of 6,8-dinitro-BIPS 7, which is exceptionally stable in DMSO even at 23°C, are shown in Figure 1.4. Generally, it is possible to obtain a reasonable absorption spectrum of the colored form by the use of a rapid scanning spectrophotometer.
The ring closed form (colorless form) has an absorption band below 400 nm, and the opened form has intense absorption in the visible region (above 350nm). The colored form of a spiropyran has characteristic properties of a merocyanine dye. The shape and position of the visible absorption bands change significantly with solvent polarity. The visible absorption maximum generally shifts to a shorter wavelength together with decrease of the extinction coefficient and broadening of the band, as the polarity of the solvent increases.2 This implies that the ground state of the colored form is relatively polar, and the polar solvents will stabilize the ground state of the colored form more so than the excited state. Concentration dependence of the colored forms in nonpolar solvents has been observed. At higher concentration, additional absorption bands and a
|
Wavelength / nm Figure 1.4. Absorption spectra of (a) the colored form (7b) of 6,8-dinitro-BIPS (2 x 10 5 M) and (b) the closed form (7a) produced by irradiation of (7b) with visible light in DMSO at 23°C. |
shoulder appear on the shorter wavelength side. This has been assigned to a dimeric species or higher aggregation species. For example, absorption band at 490 nm of the colored form of 6-nitro-BIPS in benzene increases at higher concentration (> 10-1 M)11, whereas absorption bands at 596 and 555 nm increases at low concentration. However, in a polar solvent, such as ethanol, no additional peaks have been observed.
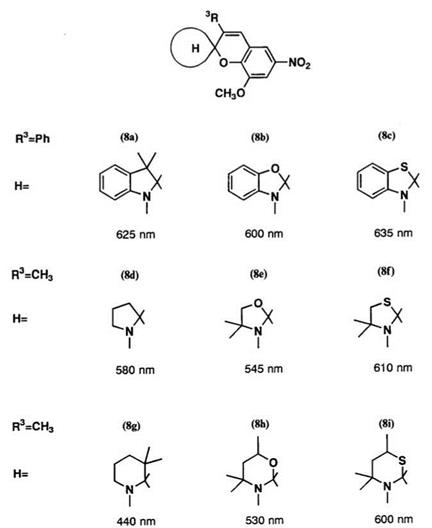
Absorption maxima for a wide range of heterocyclic systems are shown in Figure 1.5.2 When the indolyl residue 8a is replaced by other heterocyclic residue, a somewhat small shift in the Xmax occurs. Replacement with a benzothiazoline residue, 8c, results in a bathochromic shift. Comparison between saturated heterocycles 8d-8f and the corresponding benzoderiv — atives 8a-8c shows that the conjugation produced by the benzene nuclei causes a bathochromic shift (ca. 20-50 nm). Replacement of saturated five-membered heterocycles by saturated six-membered heterocycles results in a hypsochromic shift. In the case of the piperidine series (8g) a significant hypsochromic shift occurs, due to steric hindrance in the colored form.
|
Figure1.5. Xmax of the colored form for spirobenzopyrans containing various heterocyclic systems in toluene. |
Remarkable substituent effects on the absorption bands in the colored form are observed on substitution in positions 3, 6, and 8 of the spiroben — zopyran (Table 1). A nitro group at the 8-position yields a higher Xmax (~40nm) compared with a nitro group at the 6-position due to interaction of phenolate anion and oxygen atom of the nitro group. In many cases, it
Table 1. Absorption Maxima of the Colored Form of 6,8-Disubstituted
Spiroindolinobenzopyrans in Ethanol1
|
|
|
Compound |
R6 |
R8 |
R1 |
^ max, nm |
|
(9a) |
no2 |
H |
H |
532 |
|
(9b) |
H |
no2 |
H |
544 |
|
(9c) |
no2 |
MeO |
5′-Br |
550 |
|
(9d) |
MeO |
no2 |
5′-Br |
590 |
|
(9e) |
no2 |
MeO |
5′-Ph |
568 |
|
(9f) |
MeO |
no2 |
5’Ph |
625 |
|
(9g) |
no2 |
Br |
H |
533 |
|
(9h) |
Br |
no2 |
H |
570 |
|
(9i) |
no2 |
C1 |
H |
535 |
|
(9j) |
C1 |
no2 |
H |
560 |
has been shown that the steric hindrance of the substituent in 3-position causes a bathochromic shift of ^max.2 Replacement by an electron-withdrawing group, such as a nitro group, on the heterocyclic cation residue causes a bathochromic shift. The available data on other colored forms are collected in Ref. 1.
The absorption bands for both quinoid and dipolar structures have been calculated by the PPP method.2,12 The calculations for a more simplified model of the colored form of some spirobenzopyrans using the normal parameters are shown in Table 2.12 In this case, the spiro carbon in the indoline moiety is ignored in the л-electron system, and the quinoid structure is assumed.
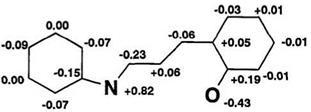
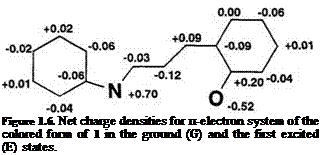
PPP calculations reproduce the nitro substituent effect and heterocyclic effect on the ^max. For example, the bathochromic shift by substitution of a nitro group is calculated (ca.20nm). It is in good agreement with the experimental value determined (Vax = 598 nm) in toluene. PPP calculation exactly predicts the bathochromic shift by benzo-annelation of the indoline and benzopyran residues (Table 2). In the neutral quinoid form, the calculated charge densities for the ground and first excited states by PPP
Table 2. PPP Calculation ofthe Colored Form of Spiroindolinobenzopyrans
![]() Compound
Compound
 H 576b 581(1.37)
H 576b 581(1.37)
NO2 598 604(1.34)
620 625(1.52)
572 577(1.39)
bln toluene.
In 1,4-dioxane.
cEi (ionization potential) = 13.6 eV, yn = 6.08 eV for N atom of indoline ring is used in PPP calculation. The ^/>3carbon for indoline component is ignored for PPP calculation. Other parameters listed in Ref. 15. ^Oscillator strength.
calculation are shown in Figure 1.6, indicating that the colored form is relatively more polar in the excited state than in the ground state.
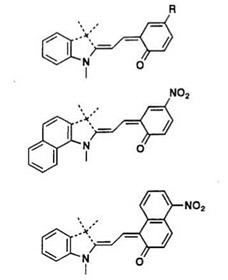
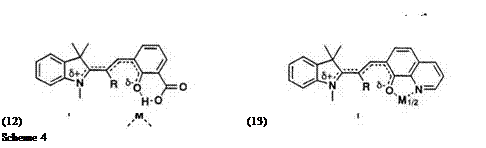
The absorption band of the colored form of many spirobenzopyrans occurs in a narrow range (ca. 550-600 nm). An approach to vary the color of the spiropyran with some limits is possible if molecular design is performed using the stable colored form. For example, cationic dye 10 is very stable and its chromophore is the conjugated polymethine, as shown in Scheme 4 (dotted line). A carbonyl group at C4 leads to a ring closure to give spiropyran containing benzopyrylium or benzothiopyrylium residue. These have been designed with the PPP method, as described in Section 1.4.1. Introduction of a л-conjugated donor group into the 2-position of benzopyran (R1) and/or replacement by a nitro-substituted group in the indoline residue (R2) is expected to produce a bathochromic shift of Xmax
(E)
 (G)
(G)
(Table 3).13 Experimentally, the extension of the n-conjugated chain resulted in a reasonably bathochromic shift, and consequently the color changes from yellow to green. But the ring-closure reaction rate significantly decreases on introduction of a conjugated donor group into the 2-position of the benzopyran ring.
|
Table 3. PPP Calculations of Benzopyrano — and Benzothiopyrano-merocyanine Dyes (10)
|
aIn CHCl2CHC12. bOscillator strength.
1.2.1.4. ‘H-NMR Spectra
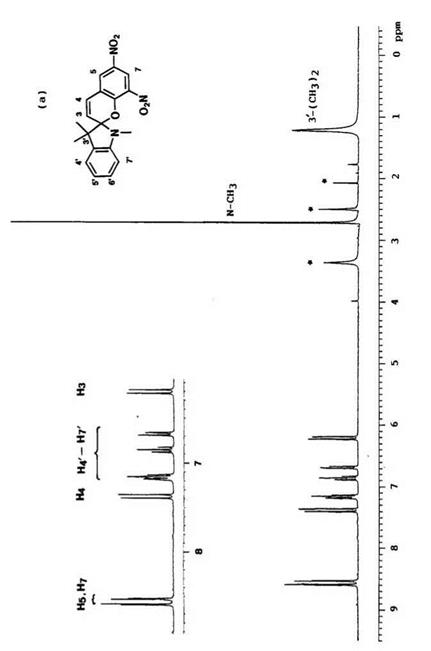
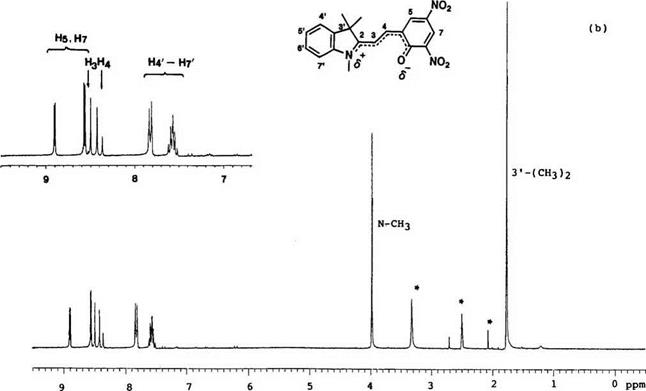
NMR spectroscopy is a convenient method for structural study of the equilibrium between the colored and the colorless form of spirobenzopyran. In the 1H-NMR spectra, the chemical shifts of gem methyl groups in 3′-position, N-methy1 group,2’11 and methine protons in 3- and 4-position are important to distinguish between the colored and colorless forms.
Typical 1H-NMR spectra (e. g., 6,8-dinitro-BIPS 7) of the colored form and colorless form are shown in Figure 1.7. The resonance peak for 3′-methyl groups in the colored form shifted to low field by 0.5 5, compared with that of the colorless form.2 Generally, for N-methy1 groups, the peak appears at ca. 5 2.7-3.0 and 4.0-4.30 in the colorless and the colored form, respectively.
|
|
|
|
The resonance peaks of two methine protons at 3,4-position are important for determining the geometry of 3,4-double bond. In the colorless form, the two methine protons appear at 5 6.0-6.4 (H3) and 7.2-7.4 (H4, J = 10 Hz) which have been assigned to the cis configuration. In the colored form, they shift to low field to 5 8.4-8.6 and 8.2-8.4 (J =15 Hz), respectively, and especially the peak of the 3-hydrogen appears at low field, due to a specific interaction with the phenolate oxygen atom.1416 Thus, a favored configuration is trans configuration C (Figure 1.1) for the colored form and other trans configuration D is possible when the colored form has an alkoxy and aryloxy group in the 3-position. The 13C chemical shifts and coupling constants for spirobenzopyrans with various heterocycles have been compiled.2
 23 июня, 2015
23 июня, 2015  Malyar
Malyar 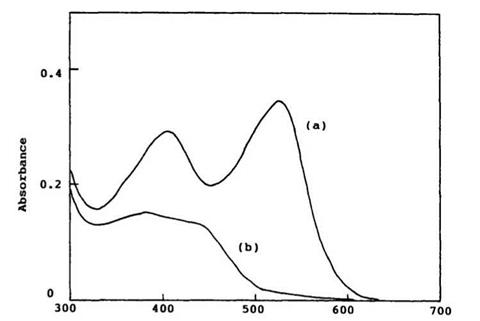
 Опубликовано в рубрике
Опубликовано в рубрике 