1.1.1. Spiroindolinobenzopyran (BIPS) and Related Series
1.1.1.1. Synthesis
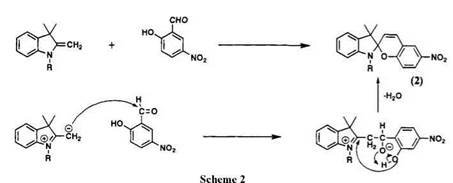
The spiroindolinobenzopyran 2 is a classical example of spiropyran and is easily prepared by the condensation of 1,3,3-trimethyl-2-methyleneindo — line (Fischer’s base) and salicylaldehyde in anhydrous ethanol or benzene (Scheme 2).1,2 The nucleophilic attack of Fischer’s base on the carbonyl group (like an enamine) gives an aldol product, which undergoes ring closure followed by dehydration. This condensation is reversible; therefore, an exchange of the salicylaldehyde component of spiropyran with a different salicylaldehyde is possible. For example, when a solution of spiropyran 2 (Scheme 2) was refluxed with 3,5-dinitro-substituted salicylaldehyde, the open form of 6,8-dinitro-BIPS was obtained.2
The condensation of Fischer’s base and salicylaldehyde does not always give spiropyran. Depending on the substituent group on salicylaldehydes, merocyanine dyes or tricyclic compounds are obtained. When a powerful electron-withdrawing substituted group, e. g., nitro group, is present in salicylaldehyde, condensation gives merocyanine dyes in benzothiazoline series,2 and an electron-donating group leads to a condensed product 3 containing three heterocycles.1
|
|
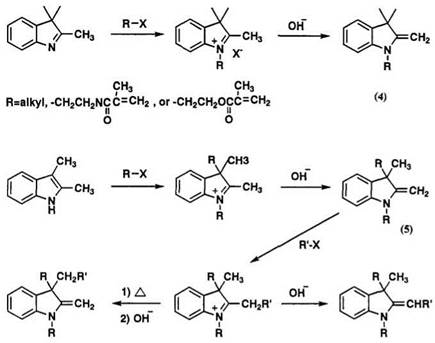
Fischer’s base, a typical starting material, is commercially available and is also obtained in situ from the corresponding quaternary salt. N-substituted indolines 4 can be prepared by N-alkylation of 2,3,3- trimethyl-3#-indole followed by alkali treatment, or by exhaustive alkylation of 2,3-dimethylindole (N — and C-alkylation) followed by alkali treatment (Scheme 3). Further, methylation of indoline 5 with methyl iodide leads to C-methylation on the methylene group or the Plancher rearrange-
|
|
ment to give 3-ethyl-substituted indoline 6.3 Reactivity of indoline 5 with higher alkyl iodide is very poor.3 However, W-(p-methylacryloyl- aminoethyl)- and ^-(P-methacryloyloxyethyl)-spiroindolinobenzopyrans
are made available for copolymers with polystyrene or poly(methylmetha — — crylate).4 Polyesters containing a spiroindolinobenzopyran are also pre-
|
Scheme 3 |
pared by condensation of bis(hydroxymethyl)spiroindolinobenzopyran with bisacid dichlorides followed by polyesterification with bisphenol A.5
Experimental Preparation of 6-nitrospiropyran 2 (R = Bu). Triethyl-
amine (2.65 g, 26 mmol) was added to a suspension of 2,3,3-trimethyl-A — butylindolinium iodide (9.0 g, 26 mmol) and 5-nitro-salicylaldehyde (4.38 g, 26 mmol) in EtOH (100 ml) under stirring. The mixture was refluxed for 2 h, and filtered off. Recrystallization from hexane gave 6-nitrospiropyran 2 (R = Bu). Also, spiropyran 2 was isolated from the filtrate, which was evaporated under reduced pressure and then was chromatographed on silica gel with dichloromethane-methanol (60:1 v /v). Total yield of 2 (8.3 g) is 88%.
 21 июня, 2015
21 июня, 2015  Malyar
Malyar 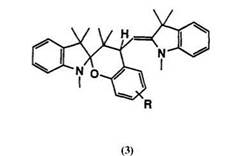
 Опубликовано в рубрике
Опубликовано в рубрике 