The spiropyran is a pyran derivative linked by a common spiro carbon atom with another heterocyclic ring. Generally, the spiropyran absorbs in the UV region, but not in the visible region. On irradiation with UV light the spiropyran undergoes heterolytic cleavage of the carbon-oxygen bond to form the colored isomer, which is referred to as the “colored form,” “merocyanine form,” or “photomerocyanine form.” The conjugation between two heterocyclic rings is made possible by this cleavage. The resulting л-extended conjugation system in photomerocyanine causes absorption in the visible region (Scheme 1). The spiropyran compounds can be regarded as the leuco form of the merocyanine dyes.
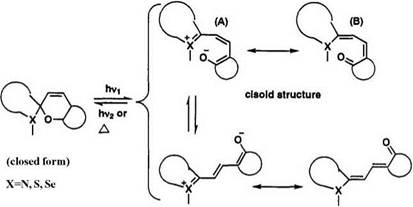
In the merocyanine form, the electronic distribution should be described by delocalization of the л-electrons with a negative charge on the oxygen and with a positive charge on the heterocyclic ring. The two important forms—(A) dipolar zwitterionic form with localized charges and (B) quinoid form, a neutral species—are regarded as the basic skeleton of merocyanine dyes. However, a better structure would be represented by a hybrid of (A) and (B) with partial charges 5+ and 5as shown in Figure
1.1.
The stereochemistry of two central double bonds in the colored form of nonsymmetrical heterocyclic systems can be represented by four cisoid
HIROYUKI NAKAZUMI • Department of Applied Materials Science, Osaka Prefecture University, Sakai, Osaka 593, Japan.
Chemistry and Applications of Leuco Dyes, edited by Muthyala. Plenum Press, New York, 1997.
|
|
|
|
|
|
|
|
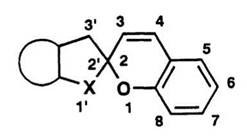
Figure 1.2. Numbering of spirobenzopyran. |
stereoisomers and four transoid stereoisomers. Typical cis-cis, cis-trans, trans-cis, and trans-trans isomers are illustrated in Figure 1.1.
The photochromism of the spiropyran depends on the structure of heterocyclic parts, the medium such as solvent or plastic films, temperature, and light energy. Though the actual mechanisms may be more complex, a simple photochromic behavior in the spiropyrans is illustrated in Scheme 1. Initially, a spiropyran is excited by photoirradiation, and then a cisoid isomer arises after dissociation of the C—O bond. Finally, the cisoid form changes to the thermodynamically stable transoid form. The equilibrium between the cisoid and transoid forms largely depends on the substituent groups. The reversal of the colored form to the colorless spiropyran occurs by thermal or photochemical energy. More detailed mechanisms will be described in Section 1.2.1.6.
The numbering of spiropyrans adopted throughout this review is indicated in Figure 1.2. The nomenclature of the spiropyran 1 is given as 1 ‘,3′,3′-trimethyl-spiro[2H-1-benzopyran-2,2’-indoline]; it is referred to as spiroindolinobenzopyran and abbreviated as BIPS.
Over the years, many spiropyran structures have been prepared. The pyran component consists of benzopyran or naphthopyran and the heterocyclic part consists of indoline, benzothiazoline, benzoxazoline, benzoselen — azoline, phenanthridine, acridine, quinoline, benzopyran, naphthopyran, xanthene, benzodithiole, benzoxathiole, and saturated heterocyclic rings such as pyrolidine and thiazolidine.
Comprehensive and important reviews on photochromism of spiro — pyrans have been published by Bertelson1 and Guglielmetti2 In the present chapter, general synthetic methods and physical properties of spiropyrans with special reference to leuco dyes will be described. The chapter is divided into the spirobenzopyran, spironaphthooxazine, and spirothiopyran and related compounds.
 21 июня, 2015
21 июня, 2015  Malyar
Malyar 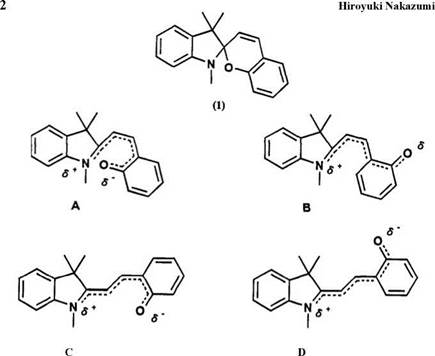
 Опубликовано в рубрике
Опубликовано в рубрике 