Water has proved to be the most harmful environment for bonded joints. Problems arise because water is universally found, and the polar groups which confer adhesive properties make the adhesives inherently hydrophilic; the substrates or substrate surfaces themselves may also be hydrophilic. Experience has demonstrated that the main processes involved in the deterioration of joints subjected to the influence of moisture are (a) absorption of water by the adhesive
(b) adsorption of water at the interface through displacement of adhesive (c) corrosion or deterioration of the substrate surface.
It would be desirable to be able to predict the strength of joints exposed to their service environments from a consideration of the likely concentration and distribution of moisture within the adhesive
Table 4.5. Parameters affecting environmental durability
Water Activity, bondline concentrations, pH and
soluble aggressive ions. If absorbed by adhesive, may plasticise and toughen.
Temperature Rate of degradation promoted by elevated
temperature; also creep effects. May aid postcuring, and may plasticise and toughen cured adhesive.
![]() Contribution to metallic corrosion and polymer degradation.
Contribution to metallic corrosion and polymer degradation.
Adhesive rheology Interfacial contact. Air Voids.
composition Chemical type affects cured structure, bulk properties, interfacial composition and stability.
cure schedule Low temperature curing implies inferior performance.
Adherends metals Surface stability.
concrete Surface dryness and coherence. Permeability. composite Moisture content. Permeability.
Surface pretreatment A most important factor. Specific to particular adherends and, sometimes, the adhesive. Nature of primer (if applicable). Bonding conditions.
Stress internal Cure shrinkage, temperature variation,
swelling by moisture.
externally applied Strained bonds more susceptible to attack.
Probably increases the rate of diffusion of an ingressing medium. Stress-corrosion. Stressed polymer, with an increased free volume, may retain more water than an unstressed polymer
Joint design Stress concentrations/tensile forces at or near
interface reveal sensitivity towards environmental attack.
![]() Duration of exposure, application of stress and adhesive viscoelasticity.
Duration of exposure, application of stress and adhesive viscoelasticity.
layer (as a function of time). Unfortunately this is not really possible(90), given the factors affecting joint ‘strength’ (Table 4.2). Nevertheless Brewis et al. (94) have observed a linear dependence of joint strength upon total water uptake for a number of bonded aluminium lap joints, and Althof(95) describes a technique for
modelling the adhesive layer stress distribution as modified by moisture uptake. Such approaches cannot take into account the effect of moisture on the interface. As an example, the theoretical water concentration profiles within the adhesive layer of one quadrant of a lap shear joint, as a function of immersion time in water at 20°C, are presented three-dimensionally in Fig. 4.21. The highly filled epoxy adhesive was cold-cured with an aliphatic amine hardener.
Mechanisms of failure. Comyn(91) suggests that water may enter and affect the performance of a bonded joint by one or a combination of the following processes. Firstly, water may enter the joint by:
(1) diffusion through the adhesive
(2) capillary transmission along the adhesive/adherend interface (wicking)
(3) capillary action through cracks and crazes in the adhesive
(4) diffusion through the adherend if it is permeable (e. g. concrete, composite).
To the above may be added osmotic pressure gradients(96).
Secondly, having accessed a joint, water may cause weakening by one or a combination of the following actions:
(5) reversible alterations to polymer mechanical properties (e. g. plasticisation, swelling)
(6) induction of reversible bondline swelling stresses
(7) irreversible alterations to polymer mechanical properties (e. g. hydrolysis, cracking, crazing)
(8) irreversible adhesive/adherend interface attack, by displacing the adhesive or by hydrating a metal adherend oxide surface.
From the foregoing discussion interfacial attack(8) is clearly the most damaging action, but adhesive plasticisation also has a profound effect on joint performance.
Diffusion and absorption of water. Water is a remarkable substance, with special properties which can be related to its molecular structure, and which govern the way its molecules interact with each other and with other substances. The water molecule’s polarity and ability to form hydrogen bonds makes it a universal solvent, allowing it to dissolve, soften or swell organic subtances whose molecules contain sufficient polar groups (especially — OH) such as epoxide(97).
|
Fig. 4.21. Theoretical water concentrations within a lap joint bondline quadrant, after immersion in water at 20 °С. Adhesive: aliphatic amine cured epoxy at 20 °С. M, = mass of water absorbed at time t. = equilibrium mass water uptake at time t = Coefficient of diffusion D = 6.6 x 10~4 m2/s (H20 , 20 °С). |
Not only can the water molecule pass through gaps in the network because of its small size, but also because it is compatible with (i. e. effectively soluble in) the polymer. Thus polar adhesives are naturally hydrophilic whereas non-polar plastics, such as PVC and Polythene, are not; i. e. ‘like permeates like’. The solubility of water in epoxies is of the order of a few mass per cent, and the coefficient of diffusion of water at 20 °С is around 10“13 m2 s-1.
For moisture to affect an adhesive joint between two metal adherends it must enter the joint by diffusion into the adhesive from an exposed edge. It is possible that moisture could ‘wick’ along the interface but this really implies either that the adhesive is displaced from the substrate at the exposed edge, or that shrinkage away from the adherend occurs. Where substrate surface corrosion takes place, the corrosion products themselves may help to strain adjacent adhesive bonds or the oxide layer itself may continue to grow at the interface, so enhancing the possibility of wicking. However wicking, as a primary mode of entry, is most unlikely and entry is normally gained by diffusion through the exposed boundary surface.
Diffusion, and adhesive displacement if it occurs, progresses inwards from the edges of the joint, and the rate of water transport through the adhesive to the interface is governed by the permeabililty (the product of diffusion and solubility). It follows that high permeability can occur with an adhesive in which diffusion is high or in which water is relatively soluble, or both. The rate of diffusion of water in an adhesive is important if water can displace the adhesive from its substrate, or if there is appreciable solubility, for this determines its rate, pattern and concentration in a bondline. If appropriate adherend surface pretreatments have not been carried out so that initial adhesion is minimal, wicking may in fact become a dominant mode of water entry. Alternatively, joint substrate surface perimeter corrosion may initiate interfacial cleavage forces and rupture the remaining adhesive bonds.
Heat-cured epoxies are often based on aromatic amines, which are less reactive than aliphatic amines, but which are much more rigid molecules (Chapter 2). This rigidity reduces their level of molecular motion and hinders water diffusion. With either type of curing agent, complete cure will lead to more cross-linking and reduced water transport. Certain fillers may also reduce permeability and water transport.
Effect of water on the adhesive. The influence of water on the adhesive is generally reversible, so that any deterioration in, say, mechanical properties is recovered upon drying. The extent of this influence depends upon the adhesive’s composition. All polymers absorb greater quantities of water when above their Tg, so that rubbery materials tend to show greater water absorption than rigid adhesives. Interestingly, the key position in structural metal-to-metal adhesives for airframe construction is occupied by epoxy-nylon adhesives, some of which display water uptakes of the order of 14%.
Water uptake by polymers is accommodated largely by swelling. For uptakes of only a few mass per cent, volumetric swelling would be of a similar or lower order(98, 99), and barely measurable. Moire fringe interferometry has been used to quantify the swelling stresses developed in a layer of adhesive upon exposure to water(lOO), and Comyn(90) describes some other work related to calculations of the stresses induced in bonded joints by water sorption.
Water depresses the Ts of adhesives. This is worrying particularly for cold-curing epoxides with typical transitions when dry in the range 40°C-50°C, and underlines the need to select adhesives whose TgS do not drop substantially with water sorption. Several attempts have been made to relate the depression of Ts to current concepts of the glass transition(90, 91). The modulus and strength of the cured polymer matrix are also lowered by water-induced plasticisation(34, 41, 95, 101, 102), in a manner akin to the organic plasticisers often used to modify the mechanical properties of adhesives. Brewis et al.(94) showed that for one particular hydrophilic adhesive/aluminium shear lap joint system, the depression of Tg could be used as a shift factor to relate the strength/temperature curve of dry joints to ones with saturated bondlines.
The fracture toughness of adhesives, other than toughened variants, generally increases with absorbed water, because of greater plastic deformation and enhanced crack-tip blunting mechanisms within a plasticised matrix(4, 28). Cohesive strength may, however, sooner or later be reduced sufficiently to offset the increased toughness. The general implication is that the toughness benefit of unmodified or initially tough products such as the rubber-modified adhesives is negated through a loss of cohesive strength(103).
The failure of a water uptake plot for thin films of adhesive (Fig. 2.22) to reach equilibrium can be taken as a sign of a chemical reaction between water and adhesive. Comyn(91) describes a number of studies of water-induced ageing processes including hydrolysis, cracking and crazing from cyclic climatic exposure, and the effect of absorbed electrolytes. Further, the hydrolysis of stressed bonds within the matrix (from internal stresses in joints, or from externally applied stresses on joints) remains a possibility.
Effect of water on the interface. The most important factor in the long term durability of bonded joints is the stability of interfacial adhesion against moisture. The absorption of water and its transport to the interface with the adherend can lead to irreversible changes, such as adhesive displacement by moisture and corrosion. Ultimately, the area of bond supporting the load diminishes until it can no longer sustain it, and joint failure occurs. The conditions leading to adhesive displacement and/or corrosion involve the nature of the adhesive bond operating in given combinations of adhesive and adherend. Brockmann(104, 105) has emphasised that the structure of the cured adhesive adjacent to the substrate surface differs from that of the bulk, because of the influence of the surface morphology and chemistry on the initial wetting and adsorption of adhesive. The inference is that this (weak) boundary layer of adhesive may be less densely cross-linked and/or have a lower concentration of filler particles than that of the bulk, and may be more susceptible to hydrolytic destruction. The rate of interfacial transport of water could also be somewhat higher than that through the bulk.
Widespread evidence indicates that the locus of failure of joints alters under the influence of moisture, from cohesive within the adhesive layer to interfacial separation. Water, in fact, displaces the adhesive when secondary valency bonds exist and in doing so applies stress to the fewer number of chemisorbed bonds which may be present. Adams and Wake(5) state that if the adhesive/adherend bond results solely from simple physical adsorption (secondary bonds), then the relative energies from adsorption of water to substrate and adhesive compared with the energy of adsorption of adhesive to substrate determine the equilibrium situation. Adhesive displacement is consequent upon a film of water existing preferentially at the interface if access is gained through defects, if the adhesive absorbs more than a few mass per cent of water, and if a high energy substrate surface is present. If the adhesive/adherend bond involves chemisorption, then displacement can only occur after hydrolytic destruction of the chemical bond.
The intrinsic stability of the interface in the presence of moisture may be assessed theoretically from the thermodynamic arguments advanced by Gledhill and Kinloch(106). Recalling the section of interfacial contact and intrinsic adhesion in Chapter 3, it is possible to deduce the thermodynamic work of adhesion, Wa, for different combinations of adhesive and substrate materials. Gledhill and Kinloch extended this to the calculation of the work of adhesion in a liquid environment, and showed that this value was negative for an epoxy/ferric oxide interface. Kinloch(69) and Comyn(91) present further predictions that bonds between epoxies and glass, aluminium, and iron and steel are unstable in water, whilst bonds to carbon — fibre composites are stable. Thus bonds to high-energy polar adherends are calculated to be unstable in the presence of moisture, and this is borne out by experience.
Joint strength rarely falls to zero but, rather, is generally lowered only. This may be because the adhesive layer has not absorbed sufficient quantities of water. It must also be recognised that the theoretical calculations assume secondary bonding only, and that chemisorption, mechanical interlocking and interdiffusion mechanisms of adhesion are not accounted for. The importance of establishing interfacial primary bonds through the use of primers and coupling agents was discussed in Chapter 3. The significance of micro-mechanical interlocking in contributing to joint durability was also discussed in that chapter, with particular regard to the micromorphology and porous oxide structures of certain metallic substrate surfaces conferred by certain pretreatments. Oxide stability was emphasised.
Other possible interfacial degradative mechanisms include the build up of osmotic pressure at the oxide/adhesive interface^) (akin to the phenomenon of paint blistering by osmotic gradients), disbonding by alkali produced by the cathodic reaction in metallic corrosion(107), and the imposition of stress leading to bond stress — corrosion cracking( 108-110).
Effect of water and salt on the adherend. Deterioration of materials such as metals and concrete is often more rapid with salt solution than with water, for example by the action of electro-chemical corrosion. Water itself may be responsible for a number of changes in the adherends; concrete is likely to get stronger with further hydration of the cement, and plastic may become weaker by plasticisation. The resin/fibre interface in composite materials is also susceptible to degradation by water. With metals, water may attack the oxide layer and/or be involved in corrosion of the bulk adherend. Stress-corrosion of adherends, especially of thin section size, needs to be considered in tandem with fatigue resistance of bonded assemblies operating under relatively high stress levels. The fatigue resistance of metals is reduced dramatically through corrosion, by loss of section or by transgranular cracking in alloyed metals.
Techniques for increasing interfacial stability
Approaches made towards improving interfacial stability were, and to a certain extent still are, more empirical than scientific. This is because the exact nature of the mechanisms by which water disrupts adhesion is unclear which, in turn, reflects a lack of knowledge about metal/adhesive bond interactions. What is clear, is that the factors affecting intrinsic adhesion and bond strength degradation are different for different combinations of adhesive and adherend surfaces. This latter observation partially explains the former statements. Thus, two areas of interest emerge, namely to employ (a) hydrophobic adhesives, with suitable composition and rheology to ensure adherend surface wetting, and (b) appropriate adherend surface pretreatment.
The composition and rheology of adhesives was discussed in Chapter 2. The rate of diffusion and solubility of water in an adhesive is important if water can displace the adhesive from its substrate. Thus adhesive formulations which are essentially hydrophobic are desirable. This may be achieved by the use of highly cross-linked polymers, polymers containing plate-like fillers, the incorporation of hydrophobic additives, and polymers possessing only enough polar groups for adhesion. Bolger(lll) discussed the balance of an optimum concentration of polar groups with polymer mobility. Mastronardi et a/.(112) showed how the presence of hydrophobic coal-tar blended with the epoxy polymer matrix of coatings strongly reduced the affinity with water, minimising water transport and maximising durability. Bowditch and Stannard(113) describe an inherently hydrophobic cold-curing epoxy for steel bonding. Coupling agents may also be mixed into the adhesive.
An enormous literature is devoted to surface pretreatments for durable bonding, as discussed in Chapter 3. Suffice it to say that chemical etching, surface conversion coatings, coupling agents and primers all have a place among the techniques. It would seem that the lowering of the surface free energy of prepared metal surfaces by the deposition of coupling agents on, or coating of, their surfaces is very important because the adsorption of water becomes unfavourable. The possibility of establishing interfacial primary bonds (chemi-sorption) of these materials to the adherends is very interesting(69, 89, 91). For mild steel, gritblasting followed by the application of certain silane primers results in greatly enhanced durability(114, 115), (see Chapter 3). Other substances which can react with metal oxides and the adhesive to form water-stable bonds were also discussed in the previous chapter. The use of priming layers should be considered carefully as they may then themselves constitute the weakest link in the joint; cohesive failure may then occur within the primer layer.
The use of sealants to coat the edges of exposed joints has some merit in constituting a barrier to liquid water, but not water vapour. Most sealants are in fact more permeable than epoxy adhesives, so that a very thick layer would have to be applied in order to be effective. The usual joint-edge adhesive spew provides a useful barrier, as well as reducing stress concentrations.
 7 августа, 2015
7 августа, 2015  Malyar
Malyar 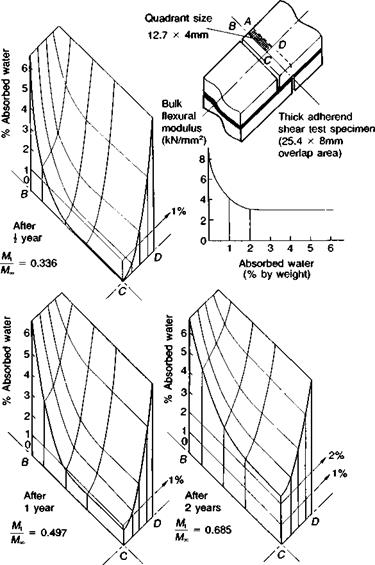
 Опубликовано в рубрике
Опубликовано в рубрике 