Some effects of surface pretreatments are summarised in Table 3.4.
Degreasing. Solvent degreasing removes grease and most potential contaminants. The choice of solvent should be based on the principle
|
|
|
|
Table 3.3. Pretreatment requirements
|
|
Table 3.4. Effects of surface pretreatments
Substrate abbreviations: M, metal; P, plastic; C. concrete. |
that like dissolves like, although toxicity, flammability and cost should be taken into consideration.
Shields (29) counsels against re-using solvent since oils and greases may simply be redistributed thereby, and a monolayer of oil
molecules suffices to constitute a weak boundary layer. Dipping in the solvent is unlikely to be successful, and the simplest form generally employed is brushing; ultrasonic or vapour degreasing systems are the most efficient. The latter enclosed systems are generally employed in mass production, in which the adherends to be degreased are suspended in a cooled zone over boiling solvent; vapour condenses on the cooled adherends and drops back into the bath so that only clean redistilled solvent makes contact. A volatile solvent such as acetone or trichloroethylene (‘trike’) should always be chosen, or else it may itself form a weak boundary layer; in other than fully enclosed systems ‘trike’ should be replaced by 1,1, 1-trichloroethane, which is much less toxic though more expensive.
For metallic substrates, alkaline cleaners and/or detergent solutions are often advised after solvent treatments, to remove dirt and inorganic solids. They may also be used instead of solvents, to obviate the safety problems such as flammability and toxicity, and should be followed by thorough rinsing and drying (in hot air) before bonding. The solvent etching of a polymer may have a similar roughening effect to the mechanical abrasion of metals(lO), although the possibility that the surface regions of a polymer may be weakened by plasticisation should not be ignored.
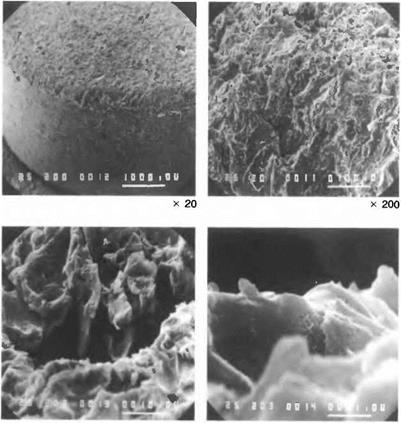
Mechanical. Mechanical treatments often cause much obvious roughening of a surface but the effect on adhesion is complex(7,ll, 16,34) and joints fabricated with highly polished mild steel adherends have shown increased strength and durability over gritblasted adherends for example. However, the real area available for adhesion is increased, the surface free energy level should be higher (because of the number of neighbourless atoms present at asperities), and the irregular profile should divert any propagating cracks into the bulk polymer. Against these potential advantages must be balanced the facts that, (a) many local interfacial stress concentrations are created, (b) proper wetting (especially by very viscous adhesives into deep narrow pits) may be rather difficult to ensure with consequent entrapment of air, and (c) segregation of the adhesive may occur with deleterious effect. If the joint is subsequently heat — cured, air trapped at the interface may rise into the bulk polymer, or else be compressed completely by the pressure of bonding in, say, an autoclave.
The various mechanical methods depend on the abrasive action of wire brushes, sand and emery papers, abrasive pads (e. g.
‘Scotchbrite’), needle guns, or shot-blasting techniques to remove unwanted surface layers; these methods are generally more difficult to control than chemical methods.
Chemical. Chemical and electrochemical treatments tend to cause more complex changes than mechanical methods. In addition to cleaning action and the removal of weak layers, chemical treatments often roughen the surface microscopically. Anodising, for example, results in a very porous surface, and other techniques for metals result in a microfibrous topography(35). Satisfactory treatments for metals must result in the formation of stable and coherent oxide, and conditions such as the duration and temperature of the process may be critical. The changes in surface geometry and chemistry will affect the rate and degree of wetting. The surface chemical change will also alter the extent of interaction between adhesive and substrate, and may produce a chemically resistant surface layer which promotes bond strength retention under adverse conditions.
A significant disadvantage of chemical methods is the toxicity of the materials used, — with a subsequent waste disposal problem.
Physical. Methods such as ionic bombardment (corona discharge) have proved successful with inert plastics, which are otherwise difficult to bond effectively because of their low surface energy (Table 3.1). It is probable that a local chemical conversion takes place on the polymer surface by oxidation, imparting a layer of higher surface free energy and thereby improving wettability (36). Allen et al.(37) report that corona discharge works quite well on aluminium and titanium surfaces, and ascribe this to the superior cleaning action, and therefore enhanced wetting, over solvent wiping. Initial lap joint strengths were, however, a little lower than obtained by employing chemically treated adherends.
 21 июля, 2015
21 июля, 2015  Malyar
Malyar 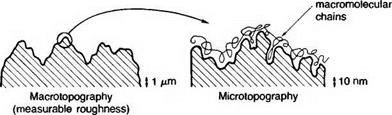
 Опубликовано в рубрике
Опубликовано в рубрике 