Water has a slow evaporation rate, much slower than solvents with a similar boiling range, Solvents such as toluene and xylene evaporate 8-10 times faster.
The latent heat of vaporisation is a significant factor when considering solvents for stoving finishes. The heat of vaporisation for water is 540 cal/g and for xylene it is 94 cal/g., hence water requires considerably more heat for vaporisation. These unconventional properties of water have to be considered when formulating coatings based upon waterborne materials. Longer flash off times between application and stoving must be introduced and stoving ovens with greater heat capacity have to be used.
The effect of humidity on the evaporation rate of water is shown in Figure 7-5.
|
% Relative Humidity Figure 7-5: The Effect of Humidity on rate of Evaporation for Water |
An increase in relative humidity does not affect the evaporation rate of xylene (xylol) but with water the evaporation rate decreases with increasing relative humidity. The vapour pressure of water increases with increasing temperature, hence the atmosphere above the coating system holds more moisture (solvent) as the temperature is increased and thus greater air flow rates are required for efficient flash off and stoving times.
 15 сентября, 2015
15 сентября, 2015  Malyar
Malyar 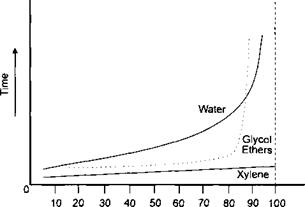
 Опубликовано в рубрике
Опубликовано в рубрике 