Azo dyes represent the largest group of disperse dyes for two reasons: (1) the ease with which an extraordinary number of molecular combinations can be generated by varying the diazo and coupling components and (2) the relatively simple process by which the dyes can be produced. With this class of dyes, manufacturers can respond much more easily to customers’ and end users’ requests for special shades and fastness characteristics.
In the following survey, the most important disperse azo dyes are divided into mono- and disazo types; then each of these classes is subdivided according to the diazo and the coupling components. The diazo component is further subdivided where appropriate into aromatic and heteroaromatic amines.
Monoazo Dyes. About 50 % of all disperse dyes are monoazo dyes, which thus represent the largest single group [9]. Relatively simple syntheses enable a range of shades from greenish yellow to cyan to be produced with this chromophore system.
With aromatic amine coupling components and carbocyclic aromatic amine diazo components, dyes of structure 1, in which aminobenzenes are the coupling components, predominate (D denotes an aromatic or heterocyclic group of the diazo component).
Of all the disperse azo dyes, this class has the greatest economic importance. Commercial products are most often represented by structure 2, in which 4- nitroaniline [100-01-6] and its substituted derivatives constitute the diazo component.
|
|
|
The most important diazo components are 4-nitroaniline [100-01-6], 2-chloro — 4-nitroaniline [ 121-87-9], 2-cyano-4-nitroaniline [ 17420-30-3], 2,4-dinitroaniline [97-02-9], 2,6-dichloro-4-nitroaniline [99-30-9], 2-bromo — [1817-73-8] and 2- chloro-4,6-dinitroaniline [3531-19-9], and 2-bromo-6-cyano-4-nitroaniline [1760194-4]. The coupling components are derived from aniline, 3-aminotoluene [10844-Г], 3-chloroaniline [108-42-9], 3-aminoacetanilide [102-28-3], and 3-amino-4- alkoxyacetanilide by N-alkylation.
This type of dye quickly became important for dyeing acetate fibers. It has been adapted to the requirements of polyester fibers and of different dyeing processes, mainly by varying the substituents R1 and R2 [11-14].
For example, the |3 — hydroxyalkyl groups (R1 and/or R2 = OH), typical of many acetate dyes, were replaced by less hydrophilic groups such as CN, OCOR, and COOR, which enhanced the affinity for polyester fibers and, in many instances, the fastness to light and sublimation [9,15].
The shade of dyes with formula 2 depends largely on the substituents X, Y, A, B, R1, and R2. If the coupling component is kept constant and X = H, variation of Y results in a bathochromic shift, which increases in the following order: H, Cl, NO2, CH3SO2, CN.
If X and Y are other than H, the situation is more complicated because of possible steric effects on the azobenzene system [16]. For example, if X = Y = Cl, steric hindrance prevents a planar alignment of the azobenzene molecule, which leads to a hypsochromic shift with simultaneous loss of clarity. This effect is exploited in the industry to produce brown shades. Cyano groups exert no steric effects, and the strongest bathochromic shifts can be obtained by using X = Y = CN; X = CN, Y = NO 2; and X = CN, Y = CH3SO2. For a given diazo component, a bathochromic shift occurs which increases in the order A = Cl, H, CH3, NHCOR. The most extreme bathochromic effect is obtained when A = NHCOR and B = OR. In combination with 2-bromo — [1817-73-8] or 2-chloro-4,6-dinitroa — niline [3531-19-9] as the diazo component, commercially important navy blue dyes are obtained; of all the monoazo dyes, these are produced in the largest quantity.
The significant influence of R1 and R2 on color is quite surprising because these substituents are not components of the chromophore system. Ester and, especially, cyano groups in these positions cause a distinct hypsochromic shift [9].
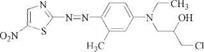
Developments in the field of heteroaromatic amines as diazo components started with the finding that 2-amino-5-nitrothiazole [121-66-4] [17] gave bright blue shades of reasonable fastness in azo dyes for cellulose acetate (e. g., 3) [18], [19]. Because of their good dischargeability but limited fastness properties, these dyes were also used on polyester. The importance of this class of dyes has diminished since some of them have been found to have sensitizing properties (see Chapter 8).
|
3 |
Blue [ 70865-21-3 ]
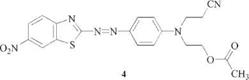
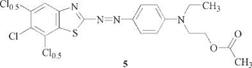
2-Amino-6-nitrobenzthiazole [6285-57-0] and 2-amino-5,6- or 6,7-dichloro — benzthiazole [24072-75-1 ], [25150-27-0] are widely employed to give scarlet to ruby shades because of their facile accessibility by ring closure of 4-nitrophe- nylthiourea (e. g., 4) [20] or 3,4-dichlorophenylthiourea, respectively. In the latter case the resulting mixture of isomers is directly utilized for diazotization and transformed into the mixture of dye isomers 5.
|
C. I. DisperseRed 177, 11122 [68133-69-7] [21] |
|
[117541-97-6], [117541-98-7] [22-24] |
Dyes with aromatic hydroxy compounds as coupling components, of which C. I. Disperse Yellow 3, 11855 [2832-40-8] is a representative example, have long been used to dye acetate fibers. Its use has been questioned for ecological reasons (see Chapter 8). For application to polyesters, sublimation fastness has been enhanced by increasing the size of the molecule.
With regard to heterocycliccompounds as coupling components, the importance of the formerly widespread pyrazolone-, aminopyrazole-, and 4-hydroxy — quinolone-based yellow azo dyes has greatly diminished with the advent of the tinctorially superior and therefore more economical pyridone azo dyes. Only a few examples have survived and then only for special applications such as for dyeing of acetate fibers. Examples are C. I. Disperse Orange 56 and C. I. Disperse Yellow5. (for structure, see Section 3.2.5).
Disazo Dyes. About 10 % of all disperse dyes are disazo compounds [9]. Even the simplest hydroxy disazo dyes, such as 4-aminoazobenzene coupled to phenol and 4-aminoazobenzene coupled to o-cresol, have a good affinity for polyester fibers and yield lightfast reddish yellow hues. However, these shades are frequently less bright than those obtained with mono azo dyes.
The introduction of an alkoxy group into the central benzene ring causes a distinct shift toward orange. A similar bathochromic effect is obtained by replacing this benzene with naphthalene.
Substitution of the first benzene nucleus by electron acceptors also causes bathochromic shifts. Disazo dyes of this type such as 6 are frequently incorporated as components of black mixtures.
|
6 |
DispersOrange 29, C. I. 26077 [19800-42-1] [25]
 16 сентября, 2015
16 сентября, 2015  Pokraskin
Pokraskin 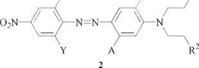
 Опубликовано в рубрике
Опубликовано в рубрике 