 |
Metal-Containing Bidentate Formazans. The most outstanding property of forma — zans is their ability to form coordination compounds with metals. They behave as monovalent, bidentate ligands and form neutral 1:2 metal chelates with divalent metals.
Opinions about the geometry of these compounds vary. A planar structure has been proposed on account of their magnetic properties [68]. However, with 1:2 Nin complexes their partial resolution into mirror-image isomers with (+)-quartz and their diamagnetism suggest a high nonplanar structure [69]. Reactions with Cun salts are dealt in [42,70,71].
Metal-Containing Tridentate Formazans. Formazans substituted with OH or COOH in the 2-position of the N1- or N5-aryl group have the same complexing properties as 2-hydroxy — (or carboxy)-2′-aminodiarylazo dyes. Similarly, they also form 1:1 complexes with four-coordinate metals, and 1:2 complexes with six-coordinate metals. Being N ligands formazans react more readily with cobalt salts than with chromium salts. The mostly blue 1:2 cobalt(III) complexes of type 34 [72] and the mostly gray-blue complexes of type 35 [73] are known.
As with the tridendate azo compounds, ligands coordinated in 6/6-rings of type 34 have a fac arrangement, and those coordinated in 5/6-rings of type 35 a mer configuration. In the formazan system, unlike the azo system, the plane of the anellated 6/6-rings in the octahedron remains almost planar. Twisting of the aromatic groups does not occur during metallization and there is therefore no loss of color intensity. Compounds of type 34 have an even larger absorption area in the UV/Vis spectrum than their metal-free precursors [72].
Tridentate formazans form 1:1 complexes with divalent metal salts:
|
|
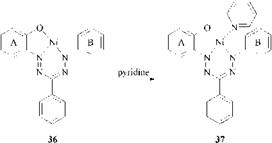
These coordinatively unsaturated complexes can undergo coordination reactions with compounds having a free electron-pair donor group, particularly pyridine, amines, ammonia, and water. Coordination is associated with a marked hyp — sochromic color change. For example, the unsaturated nickel complex36 is green and its pyridine adduct 37 is violet [74].
|
|
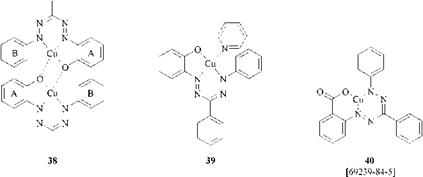
The coordinatively unsaturated complexes are even less soluble in organic solvents such as diethyl ether, benzene, and chloroform than their saturated equivalents. The magnetic moment of copper complexes of tridentate formazans with a 2-hydroxyaryl substituent at N1 is very low (1.44 pB) compared to the expected value of copper(n) complexes with an unpaired electron and strong covalent bonding. The low solubility and magnetic moment suggest that the dimerization occurs with the formation of a saturated dinuclear complex 38.
The saturated pyridine adduct 39, which does not dimerize, has a magnetic moment of 1.7 pB. The copper complex 40 has an even higher magnetic moment of 1.81 pB [75].
|
|
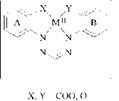
Metal Containing Tetradentate Formazans. Formazans with two complex-forming groups in the 2- and 2′-positions of the N1- and N5-aryl substituents are potential quadridentate ligands. They form 1:1 coordination complexes with four — and six- coordinate metals:
|
|
The complexes assume a planar tetragonal structure so that two monodentate ligands can occupy free coordination sites at the apices of an octahedron.
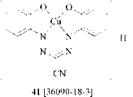
The copper complex of 1,5-bis(2-hydroxyphenyl)-3-cyanoformazan probably exists as tricyclic structure 41 because of the increased acidity of the phenolic hydroxyl group.
|
|
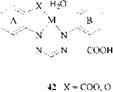
Complexes of formazans having one or two carboxylic groups in the 2- or 2,2- positions of the aryl substituents, however, appear to be bicyclic. Thus, a hydrate was obtained on reaction of 1,5-bis(2-carboxyphenyl)-3-cyanoformazan with copper acetate [76]. The coordinated water could not be removed by drying and produced a broad band in the IR spectrum at 3450 cm1. The copper complexes of formazans containing carboxyl groups were then assigned the structure 42.
|
|
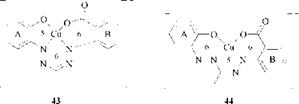
In the patent literature there are indications that the structure of copper complexes of the 2-hydroxy-2′-carboxylic acid type can be modified. After coupling and metallization, lightfastness, color intensity, and color quality can be improved by stirring the reaction mixture at low pH [77].The copper complex can be stabilized by refluxing for 15 h [78]. The coordinatively bound water may possibly be displaced by the carboxyl group in the 2-position during this aftertreatment, converting the dye to the tricyclic form 43 with a 5/6/6-membered ring system. A rearrangment may proceeds simultaneously, in the course of which the coordina — tive bond migrates from N[1] [2] to N2 of the formazan structure to give the less strained form with a 6/5/6-membered ring system 44.
|
|
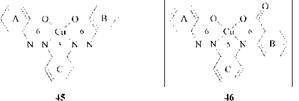
Formazan-like structures with a 6/5/6-ring system are exemplified by 45 [79] and 46 [80]:
|
|
Formazan dyes have become important reactive dyes for cotton. Formazan dyes are also used in analytical chemistry because of the high color intensity of many of their metal complexes. The chemistry of formazans was first exploited in 1962 by MacDonald to produce color photographs [81].
For fluorescence to occur, it is necessary to increase the lifetime of the first excited singlet state of the molecule so that radiative processes can compete with nonradiative processes (vibrational heat loss). This is achieved by making the molecule more rigid, thus restricting the vibrational (and rotational) degrees of freedom. In addition, a nonplanar group must also be present, otherwise the planar molecule would be pigmentary, and the excited state energy dissipated by rapid vibrational relaxation via the crystal lattice of the pigment. Indeed, this is the major reason why pigments have high lightfastness.
Fluorescent molecules span the entire region from the ultraviolet, through the visible, to the near infrared. Many dyes exhibit fluorescence, but to be of practical use, fluorescent dyes must satisfy certain requirements: they must produce a pure color dictated by their absorption and emission spectra, they must have a high molar extinction, and most important, they must have a high quantum yield. These requirements are met by very few dyes. A disadvantage shared by many fluorescent dyes is their poor lightfastness, but there are some exceptions.
The main types which find industrial use are:
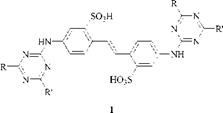
Colourless molecules with blue fluorescence are optical brightening agents that are used as blue whiteners in paper production and washing powders (see Chapter 7). The two main classes are heterocylics such as pyrazolines and particularly stilbenes (1).
|
|
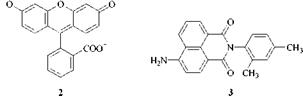
Yellow Dyes with Blue/Green Fluorescence. Fluorescein, C. I. Solvent Yellow 94, 45350:1 [518-45-6] (2), is perhaps the best known example, but equally important are coumarins, such as C. I. Basic Yellow40 [12221-86-2], and especially naphtha- limides such as C. I. Solvent Yellow44, 56200 [2478-20-8] (3).
|
|
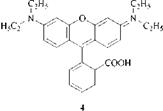
Red Dyes with Yellow/Orange/Red Fluorescence. The best known dyes of this type are the rhodamines (xanthenes), such as C. I. Solvent Red 49 [509-34-2] (4). Another type are hemicyanines, e. g. C. I. Basic Red 12, 48070 [6320-14-5] (Astraphloxine).
|
|
Blue Dyes with Red or Near-Infrared Fluorescence. These are less important than the above types and are of little importance industrially. Indeed, whilst the blue-green fluorescence of a yellow dye and the red fluorescence of a red dye increase the visual attractiveness of the dyes by making them brighter, a blue dye with red fluorescence is visually weird.
Near-Infrared Dyes with Near-Infrared Fluorescence. This type is becoming more important, particularly in biomedical applications (see Chapter 6). Phthalo — cyanines and cyanines provide this type of fluorescence.
 6 сентября, 2015
6 сентября, 2015  Pokraskin
Pokraskin 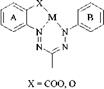
 Опубликовано в рубрике
Опубликовано в рубрике 