To maximise the electrostatic repulsion between pigment particles, it is necessary to incorporate a high concentration of ionic groupings. These are usually anionic in character which are introduced using either carboxylic or sulphonic acid groupings.
Carboxylic acids such as:
• maleic anhydride
• itaconic acid
• acrylic acid
• methacrylic acid and sulphonic acids:
• vinyl sulphonic acid
• acrylamide methyl propane sulphonic acid are the most commonly used ones.
All the above are copolymerisable with a number of backbone monomers, such as:
• acrylates
• methacrylates
• styrene
• isobutylene
• vinyl esters
• acrylamide
Of the carboxylic acids, only acrylic acid and methacrylic acid are capable of homopolymerisation and it is not surprising, therefore, that an extensive range of polyacrylates and polymethacrylates is commercially available.
Copolymer products, on the other hand, can offer additional benefits, properties such as better gloss and colour acceptance due to improved solubility in coalescents and wet edge improving solvents.
Because the monomers used are water soluble, polyacrylate dispersing agents are manufactured by redox polymerisation directly in water.
It can be demonstrated that not only the average molecular weight characteristic of a given polymer system influences dispersant efficiency, but also the polydispersity within any molecular weight range®.
|
Figure 2-7 shows the relationship between the viscosity of a pigment slurry and dosage with increasing polydispersity.
Dosage (d) |
Figure 2-7
The Effect of Polydispersal in Viscosity Dosage
The manufacture of products with such tight molecular weight control usually involves complex polymer fractionation techniques.
 16 июля, 2015
16 июля, 2015  Malyar
Malyar 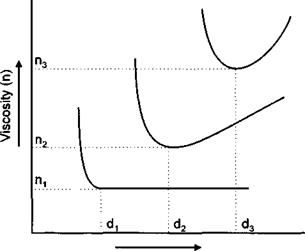
 Опубликовано в рубрике
Опубликовано в рубрике 