2.8.3.1 Sulfur Bake and Polysulfide Bake Dyes
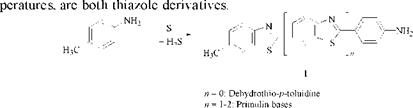 |
Research by Zerweck et al. [2] suggests that many bake dyes contain thiazole rings. It was already known that dehydrothio-j9-toluidine, which can be made by sulfur baking of ^-toluidine, and primulin bases, which are formed at higher tem-
By melting C. I. Sulphur Yellow4,53160 [1326-75-6] (prepared by sulfur melting of dehydro — p-toluidine and benzidine) with KOH, Zerweck et al. obtained not only p-aminobenzoic acid but also three distinct o-aminothiophenols 2, which they treated with chloroacetic acid and, after acidification, identified as lactams 3.
|
|
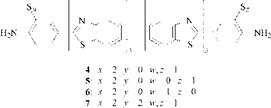
Hence, it could be inferred that C. I. Sulphur Yellow 4 essentially consists of a mixture of compounds 4-7, of which 4 is the principal constituent.
|
|
These compounds were synthesized and mixed in the calculated proportions; coloristically and in terms of fastness, the resulting mixture behaved like C. I. Sulphur Yellow4.
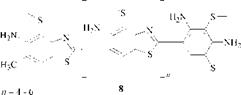
Immedial Orange C, prepared by sulfur melting of 2,4-toluenediamine, was investigated in a similar way. The results led to the conjecture that some six to eight molecules of m — toluenediamine are linked by thiazole rings, possibly in a branched form (8).
|
|
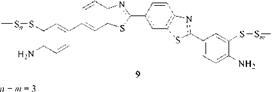
The results of Zerweck et al. were confirmed by Marek and Markovp [3], who investigated C. I. Sulphur Yellow5 [prepared (as is C. I. Sulphur Yellow 4 ) by sulfur melting of benzidine and primulin base (or dehydrothio-p-toluidine and p — toluidine), but at a higher temperature and with a longer baking time] and proposed the formula 9.
|
|
This work suggests that C. I. Sulphur Yellow 4, C. I. Sulphur Orange 1, 53050 [1326-49-4], and C. I. Sulphur Yellow5 contain thiazole (benzothiazole) rings as the chromophore.
Nothing is known of the structure of bake dyes made from nitrogen-free intermediates (i. e., from which thiazole rings cannot be formed). This group includes sulfur dyes prepared from decacyclene by sulfur baking (dry fusion process), such as C. I. Sulphur Brown 52, 53320 [1327-18-0] and also by the sulfurization of anthracene. The latter dye is thus exceptional because it is used only as a vat dye; it is largely insoluble in sodium sulfide.
The constitutional features of the sulfur-bake dyes can be summarized as follows:
1) The aromatic intermediates are combined, with the incorporation of sulfur, to form higher molecular weight chromophore systems. If the intermediate contains both CH3 and NH2 groups, thiazole rings are presumably the linking group as well as the chromophore.
2) The aromatic ring systems of the dyes in reduced form bear sodium thiolate groups.
The baking process yields chiefly yellow, orange, brown and olive dyes, depending on the intermediate.
 2 сентября, 2015
2 сентября, 2015  Pokraskin
Pokraskin 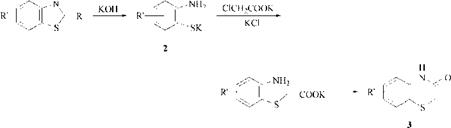
 Опубликовано в рубрике
Опубликовано в рубрике 