The precise mechanism of emulsion polymerisation remains a matter for debate. Whilst theoretical studies have given rise to the classical theories of Harkins and Smith-Ewart, commercial polymerisations do not always behave as those theories predict. There are a number of reasons for this. For example the water solubility of various monomers has a profound effect on the mechanism by which the monomer undergoes polymerisation, any one of several options being possible.
Again, where a pre-emulsion of monomer and surfactant is used (as is often the case with commercial polymerisations), the classical theories do not adequately explain the practical results.
The role of the surfactant is critical in emulsion polymerisation. A surfactant (or emulsifier) is a substance having a limited solubility in water.
A surfactant is normally a large molecule containing both an hydrophobic (water hating) and an hydrophilic (water seeking) component as shown in Figure 2-1. When added to water the surfactant orientates itself to remove the hydrophobic end from contact with water, while the hydrophilic end is hydrated by the water as depicted in Figure 2-2:
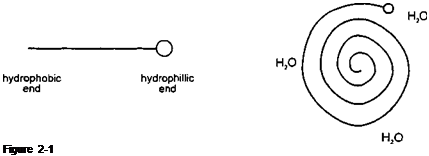 |
H20
Figure 2-2
As a certain concentration of dissolved surfactant called the “critical micelle concentration” is reached (CMC) the surfactant molecules reorientate themselves to form hydrated aggregates called micelles.
40A across. The micelles form because the surfactant molecules orientate to remove the hydrophobic part as far as possible from the water and are stabilised at the water micelle interface by the electrical charge on the polar (hydrophilic) portion of the molecule.
The major reasons for the divergence of theory (Harkins & Smith & Ewart) and practice, involve the much lower degrees of conversion of the experiments on which the academic studies were based, and the much higher concentrations of surfactant (and their method of addition) commonly employed commercially. The latter play a major role in effecting the behaviour of the micelles and the critical micelle concentration (CMC) both factors on which the classical theories were built.
Acrylic monomers added to the aqueous phase exist in three states:
a) monomer molecules can be imbibed into the micelles becoming solubilised in the process. When this occurs the micelles swell to accommodate the monomer molecules reaching sizes of 50-100A
b) monomer molecules exist in (he form of emulsified agglomerates that are partially solubilised by adsorption of emulsifier. The size of the agglomerates will depend upon the shear induced by the agitator and on the surface tension of the system. However, the agglomerates are much larger than the micelles, normally being about 10,000A ^ in diameter. The ratio of micelles to agglomerated monomer droplets is of the order 1018:1010 micelles:droplets per millilitre of aqueous phase
c) some monomer also exists as discrete molecules within the aqueous phase.
The affinity of a monomer for a particular surfactant is important. Different monomers require different surfactants.
The micelles containing monomer are dissolved in the water. However, oil soluble initiators are sometimes used, and these tend to dissolve in the monomer droplets or micelles. This confuses the prediction of the reaction in terms of the classical theories.
Before any polymerisation occurs, a dynamic equilibrium is set up between the different parts of the system.
At the start of the reaction, the initiator dissociates into free radicals, and these react with dissolved monomer forming a propagating species.
From this point theories differ as to the precise mechanism of the reaction. Smith and Ewart use three different classifications, depending upon the characteristics of the particular system. However, for the purpose of preparing industrial latices, the following is probably as good a model as any to use, assuming the required stability, conversion and film performance are obtained.
The growing species can follow a number of mechanistic routes, as indicated below:
a) the growing species can absorb emulsifier and monomer, becoming stabilised, and is able to grow until all the absorbed monomer is used up.
b) the propagating species can be absorbed into a micelle containing monomer, thereby forming a polymer particle, or nucleus, which can be stabilised by the surfactant of the micelle.
c) the monomer containing micelle can absorb the propagating species before they have absorbed sufficient emulsifier to form a stable system.
d) the growing species or free radical can enter a growing polymer particle (as outlined in options a, b and c above) resulting in chain termination.
e) the growing species can enter a particle containing polymer swollen with monomer. The growing species initiates further polymerisation in the particle, which is only terminated by absorption of further growing species.
f) the depletion of dissolved and absorbed monomer causes monomer to diffuse from the monomer droplets to replenish the equilibrium.
g) the surfactant is depleted by absorption into the polymer particles. A concentration is reached (CMC) where all the micelles disappear and further polymerisation occurs in the already formed particles. This point is typically between 15-25% conversion of monomer to polymer.
The growing species enter micelles rather than monomer droplets due to the larger number of micelles compared with droplets.
Polymerisation of droplets leads to the formation of relatively low molecular weight polymer, and also may be responsible for the formation of “hard lumps” of unstabilised polymer known as “nib’s” or “grits”.
The kinetics of addition polymerisation apply and the process can be summarised as follows:
Initiation takes place in aqueous solution and is followed by propagation in the aqueous phase. At some point the growing oligomer becomes insoluble in water and requires stabilisation. This can be achieved by the species entering a polymer particle, a monomer droplet or a surfactant micelle. Stabilisation can also occur by radical adsorption onto a surfactant molecule in solution. It is this method which is most important in the early stages of polymerisation.
The stabilised oligomer radical can be regarded as a small polymer particle and it continues to grow as monomer molecules diffuse from aqueous solution into the particle. In the early stages of its growth the polymer particle requires continual adsorption of surfactant molecules to maintain its stability.
Until it has a sheath of surfactant molecules covering its surface the particle will be unstable and can adsorb onto other polymer particles. As more polymer particles are formed, the rate of adsorption of new particles will increase until it equals their rate of formation. The period until this occurs is termed the seed stage.
Much of the controversy surrounding the mechanism is in the area of formation of particles. Some workers have claimed that particle formation does not require micelles, whilst others claim that once the micelles are exhausted particle formation ceases.
It has been shown by Smith and Ewart et al that for some systems the overall rate of emulsion polymerisation is proportional to the number of particles, the concentration of monomer and the propagation rate constant. However, the number of particles is proportional to the concentration of emulsifier and initiator.
The particle size is proportional to the number of particles and decreases exponentially with increasing emulsifier concentration (with constant surfactant type and initiator concentration) until a minimum value is reached. To reduce the particle size beyond this point other techniques have to be used.
After the seed stage is completed, the number of polymer particles remains constant, and monomer diffuses into the particles. The number of free radicals in a particle can vary with values of 0 and 1 radicals per particle being most common.
With other methods of polymerisation the rate of termination is proportional to the square of the free radical concentration. However, with emulsion polymerisation the rate of termination is controlled by the rate at which radicals enter particles. This is proportional to the number of radicals. The rate of termination is both much lower and also increases more slowly with increasing radical concentration than with other methods of polymerisation. This results in higher molecular weights and much more rapid reactions with emulsion polymerisation.
During the main propagation period monomer from the monomer droplets diffuses into polymer particles, whilst surfactant in micelles and monomer droplets is absorbed at the polymer and water interface. Where minimal surfactant concentration is used all of the surfactant is concentrated on the polymer surface. However, for surface coating applications high levels of surfactants are used and the surfactant content may be increased further by the addition of surfactant during the course of the reaction. Under these conditions micelles are likely to persist throughout the polymerisation.
Once particles are formed during the polymerisation they become swollen by absorbed monomer and as propagation continues, molecules of dissolved surfactant are absorbed. The surfactant micelles which do not participate in particle formation breakdown to form dissolved surfactant, thus maintaining the equilibrium.
The number of particles is substantially less than the number of micelles originally present (e. g. 1 to 1000). The monomer droplets are gradually depleted as the particles grow. Initiation and termination can only occur when free radicals are absorbed into the particle. A common term for particle formation is nucleation.
In addition to surfactant stabilisation, acrylic polymers may be stabilised by certain initiators which form acidic fragments which solubilise and charge stabilise the growing species.
Monomer is absorbed, imbibed, by the growing particles until all the droplets have disappeared. However, polymerisation still proceeds because the particles are swollen with unreacted monomer and only when trace amounts of monomer remain will the polymerisation rate decrease.
Consequences of the mechanisms outlined above are as follows:
a) since initiation and termination depend upon radicals entering the particle, large molecular weights can be obtained since initiation, once started continues until another radical enters the particle.
b) particle size depends upon surfactant type and concentration. This applies also where pie-emulsified reactants are used
c) the use of increased water soluble monomers will increase the rate of particle formation
d) to remove residual monomer it is preferable to use an oil soluble initiator which will be absorbed into the particle. Alternatively, 1% of a reactive water soluble
monomer or polymerisable surfactant can be added with initiator as a post reaction ‘spike’ to reduce residual free monomer
e) the particle size and surfactant system affect the viscosity, particularly if colloidal surfactant is present. The viscosity is unaffected by the molecular weight of the polymer in the latex
f) surfactant molecules are an integral part of the polymer film and are often a source of chemical and physical weakness in the performance of the coating.
Whilst the theories of Smith and Ewart have dominated academic ideas on emulsion polymerisation, they have had less of an impact on the thinking behind industrial polymerisations. These theories largely describe the formation of particles by radicals entering micelles. When no further micelles exist, then no new particles can be formed. The number of particles is controlled by the amount of surfactant required to cover the surface of the particles, and is proportional to the 0.6 power of the surfactant concentration.
The second stage of polymerisation involves particle growth, and it has been assumed that two radicals cannot co-exist within the same particle. The average number of radicals in particles is given as 0.5 and the rate of polymerisation is proportional to the number of particles.
The weaknesses of the above are the incorrect assumptions on which it is based.
Firstly emulsion polymers can be produced without surfactant by relying on the terminal sulphate group (initiator residue from persulphate) to stabilise particles, showing that micelles are not essential.
Secondly in many seed stages the number of particles reached constant value, whilst the surfactant micelles still existed.
Thirdly the theories take no account of the marked differences found in both reaction rate and number of polymer particles obtained using different surfactants.
 11 июля, 2015
11 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 