Free radicals can be produced by catalytic decomposition of a peroxide or, as illustrated below, a reducing agent can produce a Reduction-Oxidation (Redox) couple with the peroxide leading to free radical formation.
Generation of free radicals by Redox systems can occur at relatively low temperatures (even below ambient). Thus this type of initiation is of particular advantage when there are temperature constraints.
Redox systems are used to particular effect in emulsion polymerisation processes where the lower temperatures involved preclude the use of peroxides which form radicals at higher temperatures. They, are also useful where gaseous monomers (e. g. vinyl chloride, ethylene) are involved.
Redox systems are more sensitive to impurities than thermal initiators particularly in the presence of metal ions.
Typical decomposition reactions are shown below:
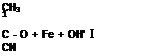 CH3
CH3
CH3— C — О — OH + Fe2* ———————— ► CH3—
CN
tertiary butyl hydroperoxide
Figure 1 -72
Multi-component Redox systems which generate both free radicals and radical ions are often employed in emulsion polymerisation processes:
S2Ob2 + Fe2+ ————— ► SO/+ S042 + Fe3+
persulphate ion radical ion
Figure 1-73
 8 июля, 2015
8 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 