Certain peroxides and azo compounds decompose when heated to give free radicals. This decomposition can occur in a single step:
CH3 CH3 CH3
I I heat I.
C6H5— С — О — О— С — CeH5 —————— ► 2С6Н5— с — о
I I I
сн3 сн3 сн3
cumyl radical
Figure 1-69
|
-*• 2C6H6— C-O II О benzoate radical |
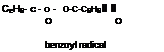 |
||
or by a two stage process:
Figure 1-70
The ratio of benzoate to phenyl radicals depends upon the reaction conditions, and in particular the reactivity of the monomers present.
Azo compounds also decompose in a similar manner, as shown by azo di-isobutyronitrile (AZDN).
|
0 1 |
0 1 |
CH3 |
0 1 |
|
I |
I |
heat I • |
heat I • |
|
-o I z |
!. z I -o I 0 1 |
—— —► 2CH3 — C — N |
——— ► 2CH3 — C + N2f |
|
CN |
CN |
CH3 |
CH3 |
 8 июля, 2015
8 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 