Indigo is readily reduced to leuco forms [cf., tetramethyl leucoindigo (8)] and oxidized to dehydroindigo (9). These reactions strongly influence the cross-conjugated system, and pronounced hypsochromic shifts of the long-wave absorption band in the UV/Vis spectrum have been measured [9]. The low frequency of the CO band in the IR spectrum of indigo at 1626 cm-1 points to the presence of hydrogen bonds (Table 2.2).
Table 2.2: IR and UV/VIS Spectra
|
|
[1] A. von Baeyer, “Zur Geschichte der Indigo-Synthese,” Ber. Dtsch. Chem. Ges. 33 (1900) Anlage IV, LI-LXX.; H. Brunck “Die Geschichte der Indigo-Fabrikation”, Ber. Dtsch. Chem. Ges. 33 (1900) Anlage V, LXXI-LXXXVI.
[2] M. Klessinger, Tetrahedron 22 (1966) 3355-3365.
[3] K. Klessinger, W. Luttke, Chem. Ber. 99 (1966) 2136-2147.
[4] H. Klessinger, W. Luttke, Tetrahedron 19 (1963) 315-335.
[5] W. Lohner, K. Praefke, “Telluroindigo”, J Organomet. Chem. 208 (1981) 35-37.
[6] W. Luttke, M. Klessinger, Chem. Ber. 97 (1946) 2342; W. Luttke, H. Hermann, M. Klessinger, Angew. Chem. Int. Ed. Engl. 5 (1966) 598.
[7] E. Wille, W. Luttke, Angew. Chemie 83 (1971) 853-854; E. Wille, W. Luttke, Liebigs Ann. Chem. 1980, 2039-2054; H. Bauer, K. Kowski, H. Kuhn, W. Luttke, P. Radema — cher, J. Mol. Struct. 445 (1998) 277-286
[8] C. Reichardt, SolventEffectsin Organic Chemistry, Weinheim, VCH, 1979, p. 193.
[9] W. Luttke, M. Klessinger, Chem. Ber. 97 (1964) 2342-2357.
 25 августа, 2015
25 августа, 2015  Pokraskin
Pokraskin 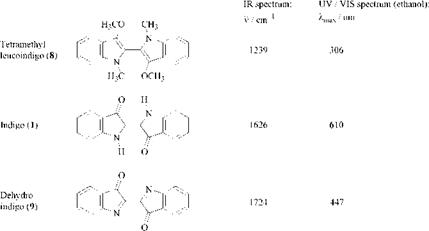
 Опубликовано в рубрике
Опубликовано в рубрике 