In most indigo syntheses the indole structure is built up by ring closure of appropriate benzene derivatives by C-N or C-C bond formation. Examples of C-N bond formation include von Baeyer’s 1878 synthesis from phenylacetic acid (3) via oxindole (4).
|
3 Oxindole (4) |
In the Heumann I synthesis, which is the basis of modern synthetic indigo production (see Section 3.5), ring closure takes place by C-C bond formation. Aniline is first treated with a chloroacetate salt to give phenylglycine salt 5, which is converted under alkaline conditions to indoxyl salt 6. Ring closure of the alkali metal salt of phenylglycine 5 gives particularly high yields in the presence of stoichiometric quantities of sodium amide. This suggests that in the first reaction step the phenylglycine salt is deprotonated to give the dianion, followed by a C-C bond formation and a 1,3-H shift to yield the indoxylate dianion 6. The mechanism is similar to that of an intramolecular (aza)-Kolbe-Schmitt synthesis. Hydrolysis, oxidation, and dimerization of the indoxylate dianion (6) then result in indigo (1) (Scheme 2.5).
|
|
 24 августа, 2015
24 августа, 2015  Pokraskin
Pokraskin 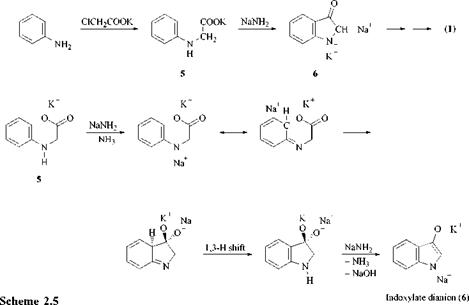
 Опубликовано в рубрике
Опубликовано в рубрике 