The benzodifuranone (BzDF) dyes are challenging anthraquinone dyes in the red shade area. The BzDF chromogen is one of the very few commercially useful novel chromogens to have been discovered in the twentieth century. As with many other discoveries [2] the BzDF chromogen was detected by accident. The pentacenequinone structure (3) assigned to the intensely red colored product obtained from the reaction of p-benzoquinone with cyanoacetic acid was questioned [3]. A compound such as (3) should not be intensely colored owing to the lack of conjugation. Instead, the red compound was correctly identified as the 3,7-diphenylbenzodifuranone (4) (A, max = 466 nm; є = 51 000 in chloroform).
|
|
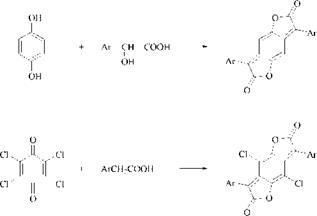
Improved syntheses from arylacetic acids and hydroquinone or substituted quinones have been devised for BzDF dyes [4] (Scheme 2.3).
|
Scheme 2.3 Synthesis of benzodifuranone (BzDF) dyes |
The BzDFs are unusual in that they span the whole color spectrum from yellow through red to blue, depending on the electron-donating power of the R group on the phenyl ring of the aryl acetic acid, i. e. Ar = C6H4R (R=H, yellow — orange; R = alkoxy, red; R = amino, blue). The first commercial BzDF, Dispersol Red C-BN, a red disperse dye for polyester, has made a significant impact. Its brightness even surpasses that of the anthraquinone reds, while its high tinctorial strength (ca. 3-4 times that of anthraquinones) makes it more cost-effective. In contrast, BzDF yellow and blue dyes have found it difficult to compete with other classes of dyes and have not found commercial use.
 23 августа, 2015
23 августа, 2015  Pokraskin
Pokraskin 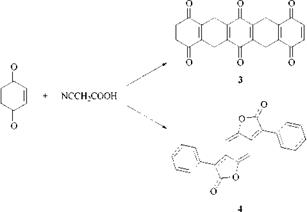
 Опубликовано в рубрике
Опубликовано в рубрике 