Addition polymerisation involves the breaking of a C=C bond and the formation of two C-C bonds. The bond energy for a C=C bond is approximately 100 Kcalories and that for a C-C bond 58.6 Kcalories.
The energy balance of the polymerisation can be crudely represented as:
(2 x 58.6) -100 = 17.2 Kcalories per mole
i. e. 17.2 Kcals of heat is liberated per mole of polymerised monomer. This equates to about 1000 kilojoules per kilogram of reacted monomer.
The polymerisation temperature greatly influences the molecular weight of the propagating chain. Hence to obtain a uniform molecular weight product it is essential to maintain a constant reaction temperature. In order to achieve this the heat of reaction must be removed from the system.
Constant agitation is essential to dissipate this, but as the degree of polymerisation increases, the viscosity of the reaction mass also increases making it more difficult to agitate the mass effectively. Agitation can be improved by diluting the reactants with an organic solvent or water, thus reducing the viscosity of the reaction mass. The diluent also has the effect of acting as a heat sink for the heat of reaction, thus helping to dissipate the excess heat.
The calculation below illustrates the temperature increase due to the reaction exotherm for a typical polymerisation.
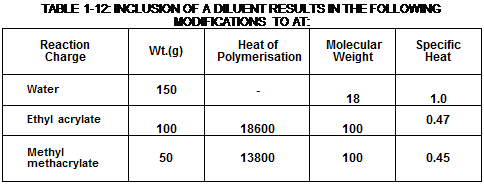
|
Monomer |
Wt.(g) |
Heat of Polymerisation (cal/mole) |
Molecular Weight |
Specific Heat |
|
Ethyl acrylate |
100 |
18600 |
100 |
0.47 |
|
Methyl methacrylate |
50 |
13800 |
100 |
0.45 |
|
TABLE 1-11: CALCULATION OF THE TEMPERATURE RISE DURING POLYMERISATION |
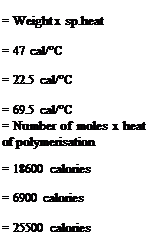 Heat capacity of reactor charge Ethyl acrylate 100 x 0.47 Methyl methacrylate 50 x 0.45 TOTAL
Heat capacity of reactor charge Ethyl acrylate 100 x 0.47 Methyl methacrylate 50 x 0.45 TOTAL
Heat evolved during polymerisation
Ethyl acrylate 100/100 x 18600 Methyl methacrylate 50/100 x 13800 TOTAL
Increase in temperature (AT) due to reaction exotherm.
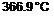 Total heat evolved _ 25500 Total heat capacity ~~ 69.5
Total heat evolved _ 25500 Total heat capacity ~~ 69.5
 |
Heat capacity of reaction charge Water 150 x 1.0 Ethyl acrylate 100 x 0.47 Methyl methacrylate 50 x 0.45 TOTAL
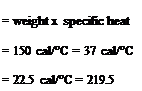
The heat of polymerisation will be unchanged at 25,500 calories. Temperature increase due to the reaction exotherm.
The effectiveness of the diluent to remove heat from the system is shown by the dramatic decrease in ДТ, from 366.9°C to 116.2°C.
Further reduction in the ДТ can be achieved by conducting the polymerisation at the reflux temperature of the diluent, the heat of reaction is used in boiling the diluent, which is then condensed in an external heat exchanger, such as a water cooled condenser, and returned to the reaction mass, in this case the “latent heat of vaporisation” of the diluent, as well as its specific heat, is utilised to remove heat from the reaction mass.
There are several methods of free radical initiated polymerisation commonly used commercially.
Two of these, solution polymerisation and emulsion polymerisation are of major importance in the manufacture of polymers for surface coating applications, and are discussed in more detail under their respective section headings.
 2 июля, 2015
2 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 