In theory, azo dyes can undergo tautomerism: azo/hydrazone for hydroxyazo dyes; azo/imino for aminoazo dyes, and azonium/ammonium for protonated azo dyes. A more detailed account of azo dye trautomerism can be found elsewhere
Azo/hydrazone tautomerism was discovered in 1884 [3]. The same orange dye was obtained by coupling benzenediazonium chloride with 1-naphthol and by condensing phenylhydrazine with 1,4-naphthoquinone. The expected products were the azo dye (62) (R=H) and the hydrazone (63) (R=H). It was correctly assumed that there was an equilibrium between the two forms, i. e., tautomerism.
|
|
The discovery prompted extensive research into azo/hydrazone tautomerism, a phenomenon which is not only interesting but also extremely important as far as commercial azo dyes are concerned because the tautomers have different colors, different properties (e. g., lightfastness), different toxicological profiles, and, most importantly, different tinctorial strengths. Since the tinctorial strength of a dye primarily determines its cost-effectiveness, it is desirable that commercial azo dyes should exist in the strongest tautomeric form. This is the hydrazone form.
Hydroxyazo dyes vary in the proportion of tautomers present, from pure azo tautomer to mixtures of azo and hydrazone tautomers, to pure hydrazone tautomer. Almost all azophenol dyes (64) exist totally in the azo form, except for a few special cases [4].
|
|
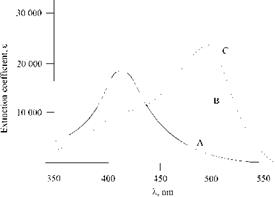
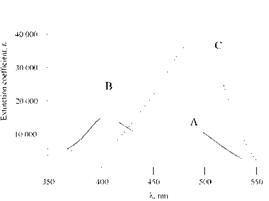
The energies of the azo and hydrazone forms of 4-phenylazo-1-naphthol dyes are similar, so both forms are present. The azo tautomers (62) are yellow (Xmax ca. 410 nm, £max ca. 20 000) and the hydrazone tautomers (63) orange (Xmax ca. 480 nm, £max ca. 40 000). The relative proportions of the tautomers are influenced by both solvent (Figure 2.4) and substituents (Figure 2.5).
|
Figure 2.4 Effect of solvent on 4-phenylazo-1-naphthol absorption. A, pyridine; B, methanol; C, acetic acid. |
|
Figure 2.5 Electronic effect of substituents in 4-phenylazo-1-naphthol (62). A, R = H; B, R = p-OCH 3;C, R = p-NO 2. |
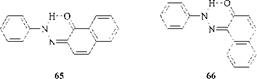
The isomeric 2-phenylazo-1-naphthols (65) and 1-phenylazo-2-naphthols (66) exist more in the hydrazone form than the azo form, as shown by their UV spectra. Their Amax values are each about 500 nm.
|
|
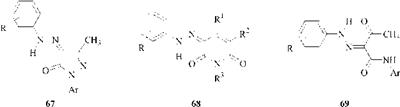
Important classes of dyes that exist totally in the hydrazone form are azopyrazolones (67), azopyridones (68), and azoacetoacetanilides (69).
|
|
All aminoazo dyes exist exclusively as the azo form; there is no evidence for the imino form. Presumably, a key factor is the relative instability of the imino group.
|
Azo Imino |
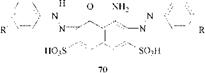
In disazo dyes derived from aminonaphthols, one group exists as a true azo group and one as a hydrazo group (70).
|
|
Aminoazo dyes undergo protonation at either the terminal nitrogen atom to give the essentially colorless ammonium tautomer (71) (Xmax ca. 325 nm),
|
71 |
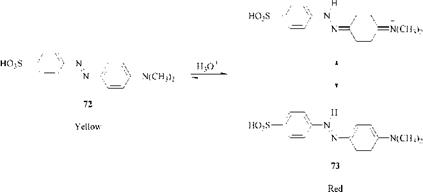
or at the |3 — nitrogen atom of the azo group to give a resonance-stabilized azonium tautomer (73), as shown for methyl orange (72). The azonium tautomer is brighter, generally more bathochromic and stronger (£max ca. 70 000) than the neutral azo dye (emax ca. 35 000). The azonium tautomers are related to diazahe — micycanine dyes used for coloring polyacrylonitrile. The most familiar use of the protonation of azo dyes is in indicator dyes such as methyl orange (72) and methyl red (Scheme 2.3):
|
|
Scheme 2.3 2.1.3.2 Metallized Azo Dyes
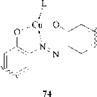
The three metals of importance in azo dyes are copper, chromium, and cobalt. The most important copper dyes are the 1:1 planar copper(n) azo dye complexes (74).
|
|
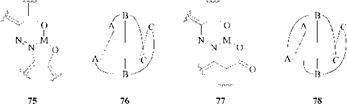
In contrast, chromium(m) and cobalt(iii) form 2:1 dye:metal complexes that have nonplanar structures. Geometrical isomerism exists. The o, o’ — dihydroxyazo dyes (75) form the Drew-Pfitzner or mer type (76) (A = C = O), whereas o-hydroxy — o’-carboxyazo dyes (77) form the Pfeiffer-Schetty or fac type (78) (A = CO 2 and C = O).
|
|
Metallization of dyes was originally carried out during the mordanting process to help fix the dye to substrate. Premetallized dyes are now used widely in various
outlets to improve the properties of the dye, particularly lightfastness. However, this is at the expense of brightness, since metallized azo dyes are duller than non- metallized dyes.
|
|
 21 августа, 2015
21 августа, 2015  Pokraskin
Pokraskin 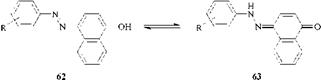

 Опубликовано в рубрике
Опубликовано в рубрике 