The term molecular weight, when applied to an acrylic polymer, lacks a single definitive description, since the molecules are not of uniform size. Every polymer sample will contain a range of molecular weights so that when presenting molecular weight data it is necessary to do so in terms of an average molecular weight or molecular weight distribution.
The term average molecular weight is ambiguous, since the “average” molecule can be defined in several ways. The most commonly encountered “average” being the number average molecular weight (M„) and the weight average molecular weight (Mw). The molecular weight of a polymer containing і repeating units (i. e. a degree of polymerisation i) may be represented as M;.
The number of molecules of weight M; in a given sample of polymer may be represented as N;.
where: щ is the number of fraction of molecules of size і in the sample.
The aggregate weight or mass of the molecules of size і may be represented as W,.
Wi
Wi = ——————
ZWi
where: wi is the mass fraction of the molecules the size і in the sample.
If W; is expressed in grams, then:
where: N is Avogadro’s Number
The Number Average Molecule Weight Mn is given by the equation:
![]() SNjMj
SNjMj
ZNi
= ZNiMi
The number average molecular weight is normally determined experimentally by measurements involving the colligative properties of the polymer, e. g. vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure.
The weight average molecular weight Mw is given by the equation:
![]() ZWjMj
ZWjMj
ZWi
= ZWiMj
The weight average molecular weight may be determined experimentally by using methods involving light scattering or sedimentation techniques.
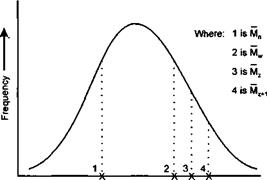
There are two other molecular weight averages commonly encountered. These are termed the “Z” average molecular weight (Mz) and the “Z + 1” average molecular weight (Mz+1). These are designed to adjust the average when there is a skewed distribution of high molecular weight fractions within a polymer sample and are denoted by the equations:
ZNiMf
and

Mn, Mw, M2 and Mz+1 may all be very different for the same polymer sample.
The relationship between the molecular weight averages is described by the molecular weight distribution.
In general: Mn < Mw < Mz < Mz+i
If the molecular weight averages are close together the polymer is said to be a monodispersed polymer and polydispersed where there is a wide separation of the averages.
In practice, a monodispersed polymer is described as one where:
77^ is between 1 and 6 Mn
and a polydispersed polymer one where:
Mw
77- is 20 or more Mn
The relationship between molecular weight distribution and the various average molecular weights can be illustrated as below:
A Gausian Distribution where the frequency is symmetrical about Mn is typified by :-
|
Molecular Weight ———- ► |
A Gamma Distribution where the frequency is asymmetrical and shows a long tail at the high molecular weight end of the distribution may be represented as :
|
Molecular Weight ———— ► Figure 1-60 |
Each molecular weight average is related to a corresponding Average Degree of Polymerisation.
ZNi і
• _ SNj I2
‘w “ £Nj і
XNj i3
lz — 2
ZNi i2
ZNi i4
iz + 1 — ї
where: in is the Number Average Degree of Polymerisation
iW(, is the Weight Average Degree of Polymerisation iz is the z Average Degree of Polymerisation iz+ris the z+1 Average Degree of Polymerisation
In general, the properties of a surface coating are dependent on the molecular weight of the polymer and change as the molecular weight and the complexity of the molecular structure increases.
Gloss retention, chemical resistance and film hardness all increase with increasing molecular weight while solubility in hydrocarbon solvents, flexibility and adhesion all increase with decreasing molecular weight.
Desired film properties may be obtained by producing a polymer of sufficiently high molecular weight. Such polymers have to be applied as solutions and form a coherent film by the evaporation of the solvent. These polymers are termed “thermoplastic” and the surface coating system is referred to as a lacquer. The lacquers are air drying or force drying systems. The final film tends to be sensitive to solvents.
Alternatively, a low molecular weight polymer can be produced containing reactive sites which film form by reaction with another polymer, chemical species or a catalyst, to form a complex high molecular weight film. These polymers are termed “thermosetting” and the surface coating system is referred to as an enamel. Thermosetting acrylics require more specific conditions for film formation, although the final film tends to be more chemically resistant, particularly with respect to solvent, than the thermoplastic types.
 30 июня, 2015
30 июня, 2015  Malyar
Malyar 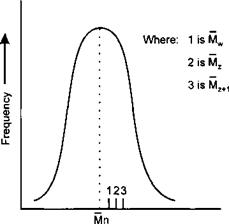
 Опубликовано в рубрике
Опубликовано в рубрике 