The azo dyes are by far the most important class, accounting for over 50% of all commercial dyes, and having been studied more than any other class. Azo dyes contain at least one azo group (—N=N—) but can contain two (disazo), three (trisazo), or, more rarely, four (tetrakisazo) or more (polyazo) azo groups. The azo group is attached to two groups, of which at least one, but more usually both, are aromatic. They exist in the trans form 1 in which the bond angle is ca. 120°, the nitrogen atoms are sp2 hybridized, and the designation of A and E groups is consistent with C. I. usage [1].
A.—N
"n—E
1
In monoazo dyes, the most important type, the A group often contains electron-accepting substituents, and the E group contains electron-donating substituents, particularly hydroxyl and amino groups. If the dyes contain only aromatic groups, such as benzene and naphthalene, they are known as carbocyclic azo dyes. If they contain one or more heterocyclic groups, the dyes are known as heterocyclic azo dyes. Examples of various azo dyes are shown in Figure 2.1. These illustrate the enormous structural variety possible in azo dyes, particularly with polyazo dyes.
|
Figure 2.1 Azo dyes. C. I. Solvent Yellow14 (2), C. I. DisperseRed 13 (3), C. I. DisperseBlue (4), C. I. Reactive Brown 1 (5), C. I. Acid Black 1 (6), C. I. Direct Green 26 (7), C. I. Direct Black 19 (8) |
 19 августа, 2015
19 августа, 2015  Pokraskin
Pokraskin 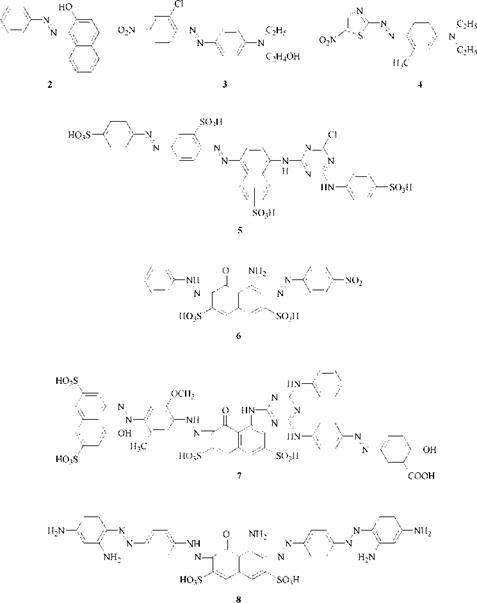
 Опубликовано в рубрике
Опубликовано в рубрике 