|
Acrylic resins containing pendant amine groups can be obtained by the use of acrylamide or methacrylamide. One method of producing pendant methylol groups on the polymer chain is by the reaction of an amide containing polymer with formaldehyde, to produce methylol groups. |
|
VyWVWWpA/V NH, NH, |
|
vywwvvywv C=0 C=0 |
|
+ 2HCHO |
|
NH NH I I CH2OH CH2OH |
|
Acrylamide containing polymer |
|
Methylolated polymer |
|
Acrylic polymer backbone |
|
Figure 1-46 The methylol groups are normally stabilised by reaction with an alcohol (usually propanol or butanol) to form an alkoxy methylol group. |
Acrylic polymer backbone AV’/V |
Both the methylol and alkoxy methylol groups will readily react with epoxy, carboxy, hydroxy and amino groups. In addition the unstabilised methylol groups readily undergo self condensation.
> 0 0 > > 0 0 >
> и 11 > > 11 11 >
S-C — NH — СН2ОН + H — О — CHj— NH— C-£—► S — C — NH — СН,—О — СН,— NH— C -4 + НгО
![]()
![]()
![]() Acrylic polymer backbone ЛДЛ/V
Acrylic polymer backbone ЛДЛ/V
Figure 1-48
The methylol groups will condense with alkoxy methylol groups
![]() о о
о о
II II
C — NH — CH2OH + C„HB— О — СН2— NH— С-
^ heat
Polymer backbone ттптш Acrylic polymer backbone
figure 1-49
The alkoxy methylol groups will also self condense under the appropriate conditions of heat and acid catalysis.
Both the methylol containing polymer and the methylol ether containing polymer will undergo similar reactions with epoxy, carboxyl and hydroxyl groups. These reactions are the basis for a major proportion of the thermosetting acrylic surface coating systems.
(xiv) Reactions of Methylol Containing Polymers with Epoxy Groups
a) Epoxy groups
|
C —NH—CH2—OH + HO—R-| ► J—C —NH —CH2—O—R-| + H20 О " ‘ О * Polymer backbone…………………….. ………………. Acrylic polymer backbone /s/s/s/s/ |
![]()
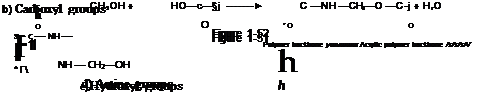 |
Acrylic polymer backbone
Figure 1-53
![]()
 26 июня, 2015
26 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 