(i) Transfer to Solvent
Here the polymerisation solvent acts as a transfer medium.
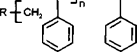
Consider the following example of polystyrene homopolymerisation in carbon tetrachloride solvent:
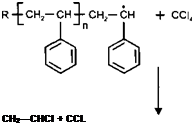 The solvent participates in the reaction resulting in the termination of the propagating chain, and the formation of a new free radical species.
The solvent participates in the reaction resulting in the termination of the propagating chain, and the formation of a new free radical species.
 |
The CC13 radical so formed, is active and may initiate polymerisation.
|
CCI3+ CH = CH2 ———- ► CH— CH2CCI3
Figure 1-7 |
The frequency with which transfer occurs will depend on the chemical structure of the solvent, the monomer and the solvent radical.
The transfer constant can be quantified in terms of the ratio of the reactivity of a given polymer radical towards the chain transfer agent and the reactivity of the given polymer towards the monomer.
|
|
where: kz = transfer constant
ks = rate coefficient for transfer to solvent
kp = rate coefficient for propagation of the polymer radicals
The kz varies with temperature and solvent type, but in general, solvents are relatively weak chain transfer agents. Typical examples of chain transfer constants are listed in the table below.
|
TABLE 1-1: CHAIN TRANSFER CONSTANTS AT 60°C
|
The term “degree of polymerisation” is used to define the number of monomer units in the polymer.
In a free radical polymerisation, where the rate of radical formation is kept constant, the degree of polymerisation is proportional to the concentration of monomer.
The degree of polymerisation, multiplied by the molecular weight of the monomer gives the polymer molecular weight. Chain transfer to the solvent not only reduces the molecular weight of the polymer formed initially, but it also affects the polydispersity which increases as the polymerisation proceeds.
Normally, when carrying out solution polymerisation on a commercial scale, solvents with chain transfer coefficients below 0.001 would be employed, unless a low molecular weight product is required.
 20 июня, 2015
20 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 