This involves the removal of the radical from the propagating chain and the transfer of the radical to another chemical species.
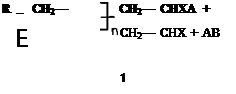 |
Both combination and disproportionation reactions result in the extinction of radicals. However, in the case of transfer reactions, the radical is not destroyed, but merely removed from the propagating chain and transferred to another species. The new radical may, dependent upon its stability, act to initiate the propagation of another polymer chain.
Figure 1-5
Transfer reactions result in the termination of a growing chain, but not the extinction of the radical.
![]()
If the radical В does initiate polymerisation, then the overall rate of propagation is unaffected by the transfer reaction. However, the molecular weight of the polymer formed under these conditions, will be lower than that formed without the occurrence of transfer reactions.
If B’ does not initiate polymerisation, it can be considered to be an inhibitor for the polymerisation, since it has, in effect, stopped chain growth and removed a free radical from the system.
There are various forms which the transfer reaction can take. It is often used as a means of modifying the length of the propagating chain, and hence, controlling the molecular weight of a polymer. In general, the qualitative effect of transfer reactions on the molecular weight of the polymer is the same, by whatever mechanism it occurs. The effect of unsaturated chain ends or residual unsaturation along the polymer backbone, is a source of chemical weakness in a surface coating system. For example, an exterior coating made from polymer with unsaturated chain ends would be particularly susceptible to degradation from UV attack.
 20 июня, 2015
20 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 