There are three types of black chromites and ferrites. These contain copper, cobalt, and nickel. The copper-containing blacks include C. I. Pigments Black 23, Black
26 (modified), and Black 28. Cobalt-containing grades include C. I. Pigments Black
27 and Black 29. There is one important nickel black, C. I. Pigment Black 30. All of these blacks have spinel crystal structures. The copper blacks are the most widely used, as they are excellent general purpose pigments. The cobalt and nickel blacks are used only in applications where their special properties are required.
Copper chromite blacks (C. I. Pigment Black 28) are spinels made from cop — per(II) oxide and chromium(III) oxide green, with a general formula of CuCr2O4. The presence of copper slightly distorts the usually cubic oxide lattice, and pure copper chromites are tetragonal spinels. The most common modifier in these blacks is manganese, which is present in many commercially available grades. Iron and molybdenum are the other modifiers of note.
Copper chromites are the most widely used CICP blacks. They offer a good jet — black color, excellent durability, and heat stability up to 1000 °C. These blacks are also excellent UV absorbers, and offer good UV opacity to the systems that employ them. Being chemically stable, copper chromite blacks are not prone to photocata — lytically decompose in the presence of titanium dioxide white. Because of this, exterior durable grays and dark shaded colors are often formulated using these blacks. High heat systems such as silicone paints and glass enamels use copper chromites to make jet-blacks and grays.
When blacks of greater heat stability than copper blacks are needed, cobalt blacks must be used. Cobalt blacks have the general formula (Cox, Fe1-x)M2O4, for x = 0 to 1, M = Fe, or Cr and Fe, and are described as C. I. Pigments Black 29 and Black 27, respectively. Nickel(II) oxide is the most commonly employed modifier. Because they contain a significant amount of cobalt, they are significantly more
|
Wavelength in nanometers Figure 5.5 Reflectance spectra of two IR-reflective CICPs compared with a non-reflective Pigment Black 28. Dips in reflection centered at 1700 and 2300 nm are due to the polymer matrix. |
expensive that other CICP blacks. The higher cost restricts their use to porcelain and glass enamel systems that require the greater heat stability.
The only important nickel blacks are described as C. I. Pigment Black 30, and have the general formula (Niy Fe1_y)(Fex, Cr1_x)2O4, where x and y range from 0 tol. The commercial grades are commonly modified with Mn(II) and Mn(III) oxides. These pigments are slightly more heat stable than the copper blacks, but not as heat stable as the cobalt blacks. They have a slight brown undertone compared to the other CICP blacks. These pigments are most commonly used for their infrared (IR) reflective properties.
In the near IR region, the nickel blacks reflect a significant amount of radiation compared with most other high performance blacks, as shown in Figure 5.5. More reflection causes less IR light to be absorbed. Exterior plastics and coatings pigmented with these blacks will therefore heat to a lesser degree in direct sunlight. Not surprisingly, the largest application for nickel blacks is in exterior coatings and PVC siding and window profiles, where durable color with minimal heating from the sun is desired.
 5 сентября, 2015
5 сентября, 2015  Pokraskin
Pokraskin 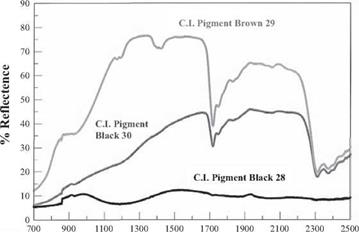
 Опубликовано в рубрике
Опубликовано в рубрике 