CICPs are metal oxides, which can have a number of possible structures. The biggest determining factor in the structure is the oxygen/metal (O/M) ratio (Table 5.1). As long as the metal ions are of a comparable size, it is the O/M ratio that largely determines what the structure will be. Two crystal structures dominate the class of CICPs — those of rutile and spinel. The hematite and corundum structures are also observed, but are much less common.
|
Table 5.1 Structures and formulas of common CICPs.
|
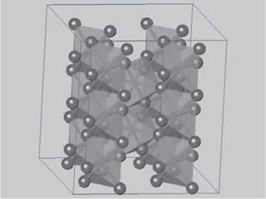
Rutile is the name of one of the crystalline phases of the mineral titanium dioxide, TiO2. It is the most dense phase of naturally occurring titanium dioxide, with a stoichiometry of 2 oxygens to 1 metal, or O/M = 2. Formulations containing metal ions of similar size to Ti(IV) with an O/M ratio of 2 will often adopt the rutile structure. CICPs with this structure contain a large fraction of TiO2 as a base oxide. Not surprisingly, rutile CICPs have many physical properties in common with titanium dioxide. Metal ions in rutile are octahedrally coordinated by six oxygen ions, as shown in Figure 5.2.
The spinel structure, named from the mineral spinel, MgAl2O4, is very common for many of the first row transition metal oxides. It has an O/M ratio of 1.33, and numerous transition metal oxides adopt this structure when they have this M/O
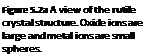 |
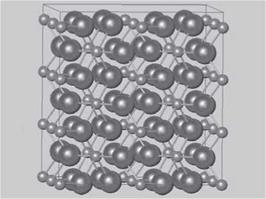 |
Figure 5.2b Another view of the rutile structure showing the connectivity of the MO6 octahedra. Metal ions, removed for clarity, reside at the center ofeach octahedron.
ratio. In fact, the structure is so stable that even stoichiometries substantially different from M3O4 can retain the spinel configuration. In these cases, the solids generally contain metal ion vacancies.
The spinel structure contains metal ions in different oxidation states. Generally, there are divalent (+2) and trivalent (+3) metal ions in the structure, although other charged ions can be accommodated. There are two distinct coordination environments for metal ions in spinel. One site is octahedrally coordinated by the oxygen ions, while the other is tetrahedrally coordinated. An example of the spinel lattice showing these sites is displayed in Figure 5.3. Normal spinels have only divalent metal ions in tetrahedral sites and only trivalent metal ions in the octahedral ones. Inverse spinels also occur, where some divalent metal ions are 6-coordi — nated, with some trivalent ions in 4-coordinated sites.
The other structures of interest are those with the basic formula M2O3, O/M = 1.5. There are two of these structures found in CICPs, one being corundum, named after the a-alumina phase of Al2O3, and the other is hematite named after the mineral Fe2O3. There is a slight difference in spatial geometry between the two structures, but both are quite similar. Metal ions are trivalent and octahedrally coordinated in both.
Figure 5.3 A view of the spinel crystal structure. Oxide ions are blue, octahedrally coordinated metals and their bonds are green, and tetrahedrally coordinated metals and their bonds are red. Some atoms and bonds have been removed for clarity.
 2 сентября, 2015
2 сентября, 2015  Pokraskin
Pokraskin 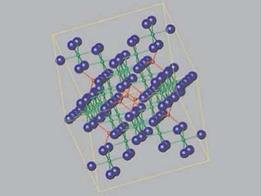
 Опубликовано в рубрике
Опубликовано в рубрике 