Cadmium compounds were first used as colorants shortly after the yellow compound cadmium sulfide was discovered by the German metallurgist Friedrich Stromeyer. The element cadmium was discovered by several chemists in 1817. Stromeyer was however the first to report it and is credited with its discovery. He recommended that the brightly colored yellow sulfide be used as an artists color.
In that same year, the Swedish chemist Berzelius identified a new element, called selenium, which was to become an essential component of cadmium reds.
While cadmium yellow pigment was at first very rare due to the scarcity of the metal, it became more available to artists in the 1830s after cadmium started to be produced commercially in Upper Silesia (now Poland). A reference to cadmium
High Performance Pigments. Edited by Edwin B. Faulkner andRussell J. Schwartz Copyright © 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 978-3-527-31405-8
Increasing ZnS
![]()
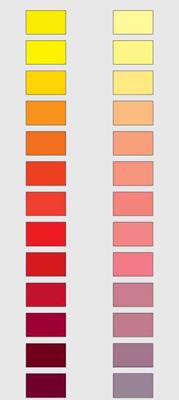 Figure 3.1 Showing mass tone and reduced tone (1 pigment: 9 titanium oxide).
Figure 3.1 Showing mass tone and reduced tone (1 pigment: 9 titanium oxide).
sulfide is made in George Field’s Practical Journal of 1809, where a yellow cadmium water color sample is discussed. Cadmium yellow was also exhibited by today’s well known Artist Supply company, Winsor and Newton, at the important Crystal Palace exhibition of 1851, in England.
In those days, cadmium pigments were expensive, but were highly regarded by artists because of their brilliance, opacity, ability to mix with other colors and, importantly, their permanence. Noteworthy was their reluctance not to darken in the presence of polluted industrial air containing hydrogen sulfide.
Cadmium yellows were also used in dyeing silks and cloths. “Steam cadmium yellow” [3] process was based on the fact that cadmium nitrate and sodium thiosulfate do not react in the cold, but produce cadmium sulfide on steaming.
Cadmium reds did not appear for some considerable time. Early reds and oranges were made by heating cadmium yellow with selenium to make red enamel pigments known as “fire red”. However, it was not until 1919 that a German patent appeared for the production of cadmium orange and red pigments [4]. These were made by heating the precipitate resulting from mixing cadmium salt solutions with alkali and alkaline earth sulfides, including barium sulfide.
The 1920s saw the development of both cadmium oil colors and lithopones, which were designed to reduce the cost of the pigment. Lithopones are virtually the same as the pure pigments in mass tone, but have considerably less tinting strength because of the large amount of barium sulfate present.
Use of cadmium pigments then grew to applications other than artists’ color and found favor in ceramic applications because of their heat stability. It was, however, their use in the rapidly expanding plastics industry in the 1950s that increased the demand for cadmiums. They exhibited excellent properties in the coloring of polymers, offering a broad range of bright, intermixable, dispersible and light fast shades. Unlike their organic counterparts they could withstand the rigorous processing temperatures demanded by engineering polymers.
 23 августа, 2015
23 августа, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 