 |
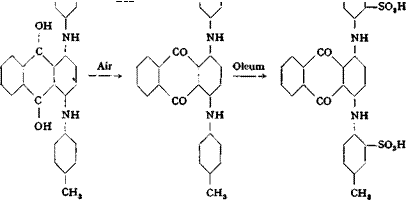
Quinizarin Green or Alizarin Cyanine Green G
In a 1.5- to 2-liter round-bottomed flask fitted with a downward condenser, stirrer, and thermometer, a mixture of 500 grams of p-toluidine and 60 grams (0.25 mole) of quinizarin (page 237) is heated to 80°C., and to it is added, with stirring, a mixture of 18 grams of boric acid, 30 grams of stannous chloride, and 16 grams of chalk. The mixture is held at 110° for 1 hour, then at 120° for 1 hour, and finally at 130° for 2 hours. During the heating, the water formed in the reaction distills off along with some p-toluidine. The reaction mixture, after cooling to 70°, is diluted with 350 cc. alcohol, cooled thoroughly, and transferred to a stoppered container, rinsing. the flask and stirrer with an additional 350 cc. alcohol. After standing overnight, the precipitated material is filtered off, stirred with 2 successive 300-cc. portions of alcohol, and finally washed on the filter with alcohol until the washings are nearly colorless.
The resulting crude base is purified by boiling it with a mixture of 70 cc. concentrated hydrochloric acid and 1600 cc. water, filtering hot, and washing the solid with hot water until it is neutral. The residue is then boiled with a mixture of 1600 cc. water and 40 cc. 40° Вё sodium hydroxide solution, and again washed until neutral. The quinizarin green base, after drying, weighs 85 to 90 grams.
Sulfonation is carried out by adding 25 grams of the base to 250 grams of 10 per cent oleum with stirring. The temperature is allowed to rise to 40-45°C. and is held at this point for 2 to 3 hours in order to get rapid solution. The mixture is then allowed to stand for 24 hours and is then poured into 1 liter water, washing out the flask and stirrer with an additional 1 liter water. The diluted acid solution, which should be at about 50-60°, is treated with 250 grams of salt. On cooling, the dye separates out in an easily filterable, crystalline form. It is filtered off and pressed out, and then redissolved in about 1500 cc. hot water. The solution is neutralized with 15 grams of soda ash and filtered through a fluted filter, washing the residue with hot water. 500 grams of salt is added to the hot filtrate, and the dye, after it has precipitated completely, is filtered off, pressed out, and dried. The yield is about 50 grains.
 |
 |
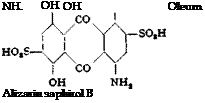
Alizarin Saphirol В and SE
|
|Na, S NHa OH
OH NHj Alizarin saphirol SE |
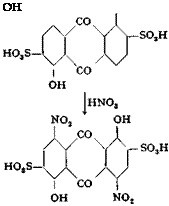
Sulfonation and Nitration. To 188 grams of 20 per cent oleum is added, with stirring at room temperature, 50 grams of thoroughly dried, finely pulverized 1,5-dihydroxyanthraquinone or its mixture with the
1,8 isomer (see page 236). The temperature is raised slowly to 100°C. during the course of 1 hour and held at this point for 2 hours more, then raised to 105° for 2 hours, and finally held at 110° until a test sample gives a clear solution in cold water. The mixture is then cooled to 25-30°, and 272 grams of 100 per cent sulfuric acid is added, followed by the dropwise addition, over a period of 1 hour, of a mixture of 36 grams of nitric acid (48° Вё) and 109 grams of 20 per cent oleum. (This mixture must be prepared very carefully, adding the oleum slowly with stirring to the well cooled nitric acid.) During the addition of the nitrating mixture, the reaction mixture is cooled in water so that the temperature does not exceed 30°C. When the addition is complete, the temperature is raised slowly to 35°, held at this point for 2 hours, then raised to 55° for 2 hours, and finally to 80° for 2 hours. The mixture is then cooled to 30° and poured, as rapidly as the foaming will permit, into
200 cc. cold water. The temperature rises to 110-115°. The resulting mixture, which is about 75 per cent sulfuric acid, is cooled and filtered, after 2 days’ standing, through a sintered glass funnel (or through asbestos ) and sucked as dry as possible. The filter cake is then dissolved in about 1 liter water, and the solution is filtered to remove any undissolved residue. The filtrate should be perfectly clear and remain so on standing.
Reduction. The nitro compound is reduced by means of a concentrated solution of sodium hydrosulfide, NaSH, the preparation of which was described on page 113. A test is made in the following way to determine how much of the reducing agent is required: 25 cc. of the filtered solution of the nitro compound is pipetted into a 750-cc. Erlen — meyer flask, diluted with 350 cc. hot water, and neutralized with soda to the point where the red coloration, which is formed, just persists. A sodium hydrosulfide solution, prepared by diluting 10 cc. of the concentrated solution to 100 cc., is then added, at 60-70°, from a burette until the color of the solution turns to a pure blue. Additional 1-cc. portions of the hydrosulfide solution are added until a definite blackening is obtained when the colorless spot on filter paper, formed by a salted-out test sample, is treated with ferrous sulfate. From the amount of hydrosulfide used in the test determination, the amount required for the total volume of the nitro solution is calculated.
The main body of the solution of the nitro compound is then neutralized with soda ash to the point giving a permanent red coloration, and heated to 60-65°C. The calculated amount of concentrated hydrosulfide solution is added slowly with stirring, and the mixture is held at 60-65° for 3 hours. Enough salt is then added to make the solution 10 per cent with respect to salt, and the solution is cooled with stirring. The precipitated dye is filtered off, washed with 15 per cent salt solution until the washings are colorless, pressed out, and dried at 90°. The yield of alizarin saphirol В is about 105 grams.
Splitting Out of One Sulfo Group. 25 grams of alizarin saphirol В is mixed with 800 cc. hot water, 46 grams of 40° Вё sodium hydroxide solution is added at 90°C., and the mixture is heated until all of the material has dissolved. A solution of 10 grams of sodium sulfide in 50 cc. water is then added slowly and the mixture is held at 95-100° with stirring, keeping the volume at about 1 liter, until a diluted test sample, when acidified with sulfuric acid, gives a filtrate which is wine red instead of blue. When this point is reached, 200 grams of salt is added immediately, and the solution is cooled with stirring. When a tempera
ture of 30-40° is reached, the precipitated dye is filtered off, washed with 10 per cent salt solution, pressed out, and dried. The yield of alizarin saphirol SE is 22 to 24 grams.
Technical Observations-. The dyes, prepared from 1,5- and 1,8-dihydroxy — anthraquinone by tfie above procedure, are so similar that frequently a mixture of the two is used. TheSE dye can be prepared without isolating the intermediate disulfo dye (alizarin saphirol B), the reduced solution from the hydrosulfide reduction being treated directly with sodium sulfide.
(b) Vat Dyes
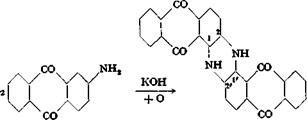
Indanthrene Blue RS from /і-Aminoanthraquinone
|
Dianthraquinonyldihydroazine or indanthrene blue RS |
An intimate mixture of 50 grams of pure /3-aminoanthraquinone (page 229), 25 grams of potassium acetate, and б grams of potassium nitrate is added in small portions to a melt of 150 grams of potassium hydroxide and 20 cc. water, heated to 210°C. A crucible made of nickel or V2A steel should be used as the reaction vessel, and the stirrer should be made of the same material. Iron is not suitable for the purpose. The additions are completed in 20 minutes, and the mixture is heated for 5 minutes more at 215-220° (not higher) with thorough stirring. The melt is then poured onto 1 kilogram of ice, washing out the residue from the reaction vessel with a small amount of water. The entire melt is dissolved in the water, and 40 grams of concentrated sulfuric acid is added to salt out the dye. The solution, which is still alkaline, is then heated to 60° and treated with 30 grams of sodium hydrosulfite. The blue leuco derivative of indanthrene blue RS which is formed is insoluble in the salt solution. When the mixture is cold, the leuco compound is filtered off, using a cotton filter on a suction funnel. The product is washed with a 2 per cent sodium hydroxide solution containing 5 grams of sodium hydrosulfite per liter until the washings are clear and light blue in color. The precipitate is then stirred with 500 cc. water
at 60°, and a stream of air is blown through the mixture until all of the leuco derivative is oxidized as shown by the insolubility of a small test sample in a large volume of water. The dye is filtered off, washed with water, and dried. The yield is about 22 grams.
Indanthrene blue RS dyes cotton a pure, deep blue from its blue hydrosulfite vat. The dye has exceptional light fastness. Its fastness to chlorine is only moderate, but this can be improved by chlorination. This chlorination can be effected by the action of chlorine on the sulfuric acid solution in the presence of sodium nitrite (GCD brand), by the action of sulfuryl chloride on the nitrobenzene solution (BCS brand), or by the action of chlorine in chlorosulfonic acid, possibly in the presence of a carrier such as iron chloride or antimony pentachloride.
All efforts to increase the yield of indanthrene blue to more than 45 per cent of the theoretical amount have so far been unsuccessful. By-products are always formed, among them alizarin and many others. In addition, some of the dye is destroyed in the fusion.
The recently published[62] [63] preparation from l-chloro-2-aminoanthraquinone by heating with cuprous iodide (Ullman-Goldberg reaction) gives still less satisfactory results.
The chlorine fastness of indanthrene blue varies directly with the purity of the aminoanthraquinone used. Also, the finished dye can be purified by treating it in concentrated sulfuric acid solution with an oxidizing agent (manganese dioxide, etc.) which destroys the impurities. Such purified dyes are marketed as indanthrene brilliant blue. They are somewhat stronger, and have better chlorine fastness, than the original dye. The use of potassium acetate was first proposed by Pope[64] who also pointed out the advantage of using an oxidizing agent in addition to the acetate (or formate)■ It is essential that the fusion is not carried out at too high a temperature or for too long a time. Hence, in the plant only small batches are run, e. g., about 20 kilograms. The reaction vessels are either pure nickel or stainless steel.
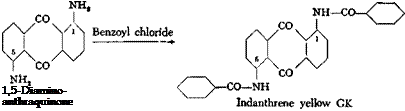 |
Indanthrene Yellow GK[65]
To 1,5-diaminoanthraquinone (page 237) in 20 parts of 1,2-dichlorobenzene (or nitrobenzene) is added slowly at 140° the calculated amount of benzoyl chloride. Hydrogen chloride is generated (hood!). When the gas evolution has ceased (about 1 hour), the mixture is cooled and the dye is filtered off. The yield is quantitative.
From its weakly alkaline hydrosulfite vat at 45°C., 1,5-dibenzoyIaminoanthra — quinone gives yellow shades with excellent characteristics. The designation К signifies that the dye is one which must not be used at too high a temperature or it is destroyed (Kaltkuper).
In an analogous manner, indanthrene red 5 GK is prepared by benzoylation of 1,4-diaminoanthraquinone (page 232), and algol yellow WG by benzoylation of 1-aminoanthraquinone (page 231).
 15 декабря, 2015
15 декабря, 2015  Pokraskin
Pokraskin 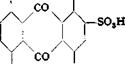
 Опубликовано в рубрике
Опубликовано в рубрике 