The solution from (b) is mixed with 45 grams of sulfuric acid (66° Вё) and 100 grams of diethylaniline, and boiled under reflux for 2 days. The mixture is then made strongly alkaline by the addition of about 100 grams of 30 per cent sodium hydroxide solution, and the excess diethylaniline is driven off with steam. The alkaline residue is filtered if necessary and made distinctly acid by adding 50 grams of concentrated sulfuric acid. The inner salt of the leuco compound separates, in the course of 24 hours, in the form of fine white needles. These are filtered off and
* The Weldon mud (“Manganschlamm”) is calculated as MnOa, i. e., one uses the equivalent of exactly 100 grams of manganese dioxide. Weldon mud, a by-product of saccharin manufacture, has the approximate composition, МП3О4.
washed thoroughly with water. After drying at 80°C., the material weighs about 70 grams.
(d) Oxidation to the Dye
This oxidation resembles closely that for malachite green. 50 grams of the leuco compound is dissolved in hot water containing 8 grams of soda ash, the material being quite insoluble in cold soda solution. The solution, which should be exactly neutral to litmus, is made up to 1000 cc. and cooled to 0°C., and to it is added, in one portion with vigorous stirring, a mixture of 15 grams of concentrated sulfuric acid and a paste containing 22 grams of lead peroxide (see page 138). The mixture is held at 0-5° for 1 hour and is then heated to 80° and filtered to remove the lead sulfate. The filtrate is evaporated to 600 cc., preferably in vacuo, and 50 grams of salt is added. In the course of a day, the dye crystallizes out and is filtered off and washed with a small amount of saturated salt solution. The dye is dried in a small porcelain dish after adding a few drops of concentrated ammonia to neutralize traces of mineral acid. The yield of dye is about 32 grams.
If the volume of the filtrate after removing the lead sulfate is not greater than 1 liter, the dye can be salted out directly, without previously evaporating the solution, by adding 20 parts of salt. (Salting out should be tried first in a test tube.)
In the presence of acid, the solution of the dye is green; in this case, enough soda is added to change the color to blue before the salting out operation.
Technical Observations. Benzaldehydedisulfonic acid is so soluble that it cannot be isolated. The oxidation is carried out in large steam jacketed kneading vessels which are sufficiently strong in construction to permit continuous stirring throughout the reaction. Also, the operation can be done with less sulfuric acid as a diluent. The liming and steaming are carried out by standard methods, except that some difficulties are encountered in that the heating pipes rapidly become encrusted with calcium sulfate. It is not possible to add enough soda to precipitate the calcium completely because of the sensitivity of the aldehydedisulfonic acid. The condensation reaction is carried out in leaded kettles and the oxidation in wooden vats equipped with propeller stirrers made from ash wood. The dye solution is evaporated in vacuo, and the dye is always removed by centrifuging. The mother liquor is treated with aniline to precipitate a second crop of eye which is less pure and sold as second quality.
More recently, benzaldehydedisulfonic acid has been prepared, not from toluene, but from toluenesulfonyl chloride. This use, as well as others, has considerably increased the price of the previously ahnost worthless p — toluenesulfonyl chloride.
|
SOj- |
Cl |
SOsH |
|
|
Hydrolysis with |
/ I I |
+ SO, |
|
|
s) |
cone. HjSO^ |
к/ |
6o% ’ |
|
CH, |
CHS |
|
p-Toluenesul- fonyl chloride |
|
acid |
SO. H
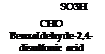 S03H CH,
S03H CH,
Toluene-2,4- disulfonic acid
The toluenesulfonyl chloride can first be sulfonated instead of hydrolyzed. The hydrolysis then taxes place with the formation of chlorosulfonic acid, and when water is added after the sulfonation, hydrochloric acid is generated immediately.
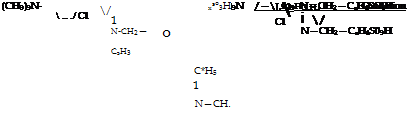 |
Wool Blue 5B
(CH3)2N—/ -C—OH
ч — Л
Cl 1
N—CH2
C2H,
A mixture of 9.2 grams (0.05 mole) of o-chloro-p-dimethylamino — benzaldehyde (page 119), 32 grams of ethylbenzylanilinesulfonic acid,
and 225 grams of 12.5 per cent sulfuric acid is boiled under reflux with stirring for 24 horn’s. The leuco compound is then precipitated as its sodium salt by careful neutralization with sodium hydroxide. The compound separates as a gummy mass. The mother liquor is decanted, and the residue is dissolved in 500 cc. water. The solution is mixed with an equal volume of saturated salt solution. The leuco compound is precipitated as white flocks which are filtered off, washed with 10 per cent salt solution, and dried in vacuo at 100°C. The yield is 36 grams of leuco compound, or 91 per cent of the theoretical amount.
The leuco compound (7.9 grams, 0.01 mole) is dissolved in 100 cc. 50 per cent acetic acid, and to the well stirred solution are added, simultaneously, 30 cc. 10 per cent oxalic acid solution and 12.5 cc. 10 per cent sodium bichromate solution. The mixture turns blue immediately and the oxidation is complete in 10 minutes. The solution is mixed with an equal volume of saturated salt solution and the acetic acid is neutralized with ammonia. The dye precipitates as a reddish, bronzy slime which soon becomes glass-hard. The product is dissolved in hot water, the solution is filtered, and the dye is then salted out by adding an equal volume of saturated salt solution. In this way, 7.8 grams of pure dye is obtained. It gives bright blue tints on wool from neutral or weakly acid solution. The dye is not very fast to light.
Victoria Blue В
N(CH$),
|
|
Michler’s ketone (page 139) is treated with 25 per cent of its weight of toluene and mixed with an equimolar amount of phenyl-a-naphthyl — amine (page 179). 1 mole of phosphorous oxychloride is then added, and the mixture is stirred until it becomes thick. The temperature should rise to about 75-80°C. After about 45 minutes, the mass becomes so thick that it can no longer be stirred. It is then mixed with 10 parts
of water, heated to boiling to decompose the phosphoric acid addition product, and treated with enough sodium hydroxide solution to make the mass green and lustrous. The toluene is driven off with steam, and the mother liquor poured off. The dye is dried at 80-90°. The yield is quantitative.
Victoria pure blue BO is prepared in a similar way from the tetraethyl ketone and ethyl-a-naphthylamine.
 |
 |
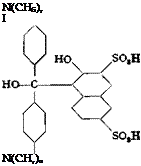 |
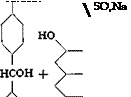
Wool Green S
N(CH,)a
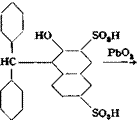
0.05 mole (13.5 grams) of tetramethyl-p. p’-diaminobenzohydrol (page 138) is added with stirring to 120 grams of sulfuric acid (66° Вё) at such a rate that the temperature does not rise above 40°C. When the solid has all dissolved, the solution is cooled in an ice bath to about 5°, and the amount of technical R salt corresponding to 21.8 grams (0.0625 mole) of pure sodium 2-naphthol-3,6-disulfonate is added with stirring while the temperature is kept at 5-10°. The mixture is then stirred at room temperature for about 2 hours, then warmed to 60° during the hext hour, and held at this temperature for about 2 hours until a test sample, diluted with water and treated with sodium acetate, gives only a weak blue coloration which is not increased by heating. The reaction mixture is then poured into 700 cc. cold water, and crystallization of the condensation product is started by seeding or scratching the walls of the vessel with a glass rod. Stirring is continued for several hours, and the mixture is allowed to stand overnight. The sandy precipitate is filtered off, washed with cold water until the washings are no longer acid to Congo red, then twice with alcohol and twice with ether, and finally dried in a vacuum desiccator. The yield of loose, gray white powder is 24 grams, or about 86 per cent of the theoretical amount.
The leuco compound is dissolved in 480 cc. cold water containing 15 grams of soda ash, and the solution is cooled by addition of 160 grams
of ice. With vigorous stirring, lead dioxide paste, prepared from 14.3 grams of lead nitrate (page 138), is added in one portion. The mixture immediately becomes deep blue. Stirring is continued for 30 minutes at room temperature, and the mixture is then heated to 80°C. over a period of 1 hour, after which the lead carbonate is removed by filtration and washed with hot water. The filtrate is cooled and acidified with 20 cc. concentrated hydrochloric acid,* and 200 grams of salt is added with stirring. The bronzy, crystalline precipitate of the dye is filtered off after standing overnight, washed with salt solution, and dried, preferably at 50-60° in vacuo. The product, which contains some salt, weighs about 24 grams.
Wool green S dyes wool from acid baths to give very strong blue green shades. This dye and naphthalene green V, which is prepared in an analogous manner from tetraethyldiaminobenzohydrol and 2,7-naphthalenedisulfonic acid, are among the most widely used green wool dyes.
 6 декабря, 2015
6 декабря, 2015  Pokraskin
Pokraskin 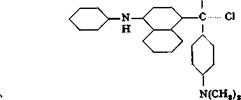
 Опубликовано в рубрике
Опубликовано в рубрике 