Benzidine can be combined with all of the phenols and amines which are commonly used in preparing azo dyes. It happens that only one of the two diazo groups in tetrazobenzidine is highly reactive while the other is rather inactive. It is possible, therefore, to make not only the benzidine dyes employing two molecules of a single phenol or amine coupling component, but also, in many cases, the so-called “mixed” benzidine dyes. The mixed dyes are formed, however, only when the first coupling component is not too reactive so that the disazo dye is not formed immediately from the intermediate compound:
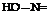 -N—N—X
-N—N—X
It would lead much too far afield to discuss even the most important variations of these intermediates, so only a few examples will be mentioned. Of special importance is the intermediate formed by monocoupling tetrazotized benzidine with salicyclic acid. This product is formed in high purity, since a second coupling reaction with another molecule of salicyclic acid can be made to take place only with difficulty, by using an excess of sodium hydroxide, whereas the first coupling takes place in soda solution. Furthermore, no difficulties are encountered in coupling tetrazotized benzidine with one molecule of the monoazo dye from p-nitroaniline and H acid, or with one molecule of H acid itself in mineral acid solution. Both of these latter reactions are discussed in more detail later.
 2 декабря, 2015
2 декабря, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 