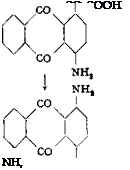
The preparation of 1-aminoanthraquinone is quite similar to that of the 2 isomer, except that the sulfonation is carried out in the presence of mercury salts which direct the sulfo group to the a position to the extent of 98 per cent.
NH,
/ + Hg salt k J ) + Na nitroben
![]()
 |
||
x Co’ zenesulfonate qq
(a) Anthraquinone-l-sulfonic Acid
An intimate mixture of 208 grams of pure, dry anthraquinone and 4 grams of elutriated mercuric oxide or mercuric sulfate is added, with
78 See also, Geigy, Ger. Pat. 347,683 (1921) [Frdl, 13, 398 (1916-1921)1.
stirring, to 200 cc. oleum containing 20 per cent SO3. The mixture is stirred at 50°C. until solution is complete, and then the temperature is raised to 130-135° in the course of 1 hour. During the next 2 hours, 50 grams of 60 per cent oleum (or the equivalent quantity of oleum of a different concentration) is added dropwise, and stirring is continued for 1 hour more at 135°. The mixture is then cooled and poured into 2 liters ice water. The diluted mixture is heated to boiling and filtered to remove unreacted anthraquinone (about 60 grams). The filtrate is heated to boiling and treated with a hot solution of 80 grams of potassium chloride in a small amount of water. The potassium salt of anthraquinone-1-sulfonic acid separates in beautiful crystals. The mixture is allowed to cool to 60° with stirring and held at this temperature for 6 hours. It is then filtered through a warm funnel, and the precipitate is washed with saturated KC1 solution, then with a small volume of water, and dried. The yield is about 190 grams, or 58 per cent of the theoretical amount.
An additional 15 to 20 grams of less pure material is obtained by cooling the mother liquor. This fraction is mixed with some of the potassium salt of the 1,5- disulfonic acid which is very soluble at 60°, but rather difficultly soluble in the cold.
(b) 1-Aminoanthraquinone
A mixture of 60 grams of potassium anthraquinone-l-sulfonate, 120 grams of 24 per cent ammonia, and 21 grams of sodium m-nitrobenzenesulfonate is placed in a rotating autoclave. The temperature is raised to 170-175°C. over a period of 4 hours and held at this point for 12 hours. The pressure is about 24 to 27 atmospheres. After cooling, the contents of the autoclave are filtered, and the precipitate is pressed out strongly and washed with a small volume of hot water. The solid is then placed in 300 cc. boiling water to which some hydrochloric acid has been added, and the mixture is filtered hot, washing the residue thoroughly with hot water. The product thus obtained is a technically pure grade of 1-aminoanthraquinone melting at 238°. The yield is about 39 grams, or 95 per cent of the theoretical amount. Crystallization from xylene yields the amine in the form of small red black prisms with metallic reflex, melting at 241°. The yield of pure amine is about 75 per cent of the theoretical amount.
(e) 1-Anthraquinonyloxamic Acid19
In an agitator kettle containing a few steel balls (similar to the one
79 Noelting and Wortmann, Ber., 39, 642 (1906). Curtis, Dissertation, Zurich* Weida, 1929.
described on page 87), a mixture of 44 grams of 1-aminoanthraquinone and 132 grams of anhydrous oxalic acid is heated in an oil bath to 115-120°C. (internal temperature), with stirring, until the originally red melt has become brownish yellow and resolidified. This requires about 1.5 hours. The reaction is completed when a test sample, extracted with water, shows the correct melting point of 224-226°. The mixture is treated with 350 cc. hot water, boiled for 30 minutes, filtered hot, and the residue is washed with hot water and dried. About 57 grams (98 per cent of the theoretical amount) of the oxamic acid, melting at 224-226°, is obtained.
(d) 4-Nitro-l-anthraquinonyloxamic Acid
The oxamic acid from (c) (57 grams) is dissolved in 570 grams of sulfuric acid (66° Be), and the solution is cooled to 0°C. With good stirring, 21.7 grams of finely powdered potassium nitrate (dried at 120°) is added, maintaining a temperature of 0-2°. Stirring is continued for 6 hours at 0°, and the mixture is placed in an ice chest overnight. It is then poured onto 2 kilograms of ice, and the precipitate is filtered off, stirred with 2 liters water, filtered again, and washed thoroughly with cold water.
( e) 1,4-Diami noant hr aquinone
The moist filter cake from (d) is mixed with 600 cc. water, the mixture is heated to boiling, and enough soda is added carefully to make the mixture definitely alkaline. The brown flocculent precipitate changes to a resinous mass. 200 grams of crystalline sodium sulfide is added, and the mixture is boiled under reflux for 2 hours. In this treatment, the nitro group is reduced and the oxalic acid residue is split off. The mixture is filtered hot, and the precipitate is washed with hot water until the washings are nearly colorless. Contrary to the usual procedure, in this case the wash liquid is not sucked out completely each time and full suction is applied only after the final wash. The dried 1,4-diamino- anthraquinone weighs about 45 grams (about 95 per cent of the theoretical amount based on 1-aminoanthraquinone) and melts at 260-265°.
Technical Observations. Indanthrene red 5 GK is formed by benzoylation of 1,4-diaminoanthraquinone, and indanthrene yellow GK by benzoylation of the 1,5 isomer.
1-Aminoanthraquinone is used not only in the preparation of 1,4-diaminoanthraquinone; it is the starting material for a series of important anthraquinone dyes of which alizarin pure blue В is an example. The preparation of this dye is briefly as follows:
1 — Aminoanthraquinone in 10 parts of glacial acetic acid is treated for 2 days at 50°C. with 2.5 moles of bromine. The reaction mixture is poured into bisulfite solution to destroy the excess bromine, yielding l-amino-2,4-dibromoanthraquinone,
m. p. 222° (from glacial acetic acid). One mole of this product is dissolved in 7 parts of dry p-toluidine, and the solution is heated to 200° with exactly 1 mole of anhydrous sodium acetate. Some of the p-toluidine is distilled off to carry out all of the water. When the reaction is completed, the mixture is diluted with an equal volume of alcohol, filtered, and the product is washed with alcohol. A yield of about 82 per cent of l-amino-2-bromo-4-p-toluidinoanthraquinone is obtained. This base is dissolved in 6 parts of 100 per cent sulfuric acid at 25°, and about 1.5 parts of 66 per cent oleum is added carefully over a period of 1 hour. The temperature must not exceed 45° or the dye is destroyed. When a test portion is completely soluble in soda solution, the reaction mixture is poured into a large volume of water, and salt is added to make the solution 10 per cent with respect to salt. The precipitated dye is filtered off, washed with 10 per cent salt. solution, and dried at 90°. The yield is quantitative.
|
|
Alizarin pure blue dyes a pure blue quite similar to the color of aniline blue (triphenylrosaniline). Its color strength is high and its fight fastness is very good. It is especially valuable for tin phosphate weighted silk with which it is widely used.
If an amine is used, instead of ammonia, to react with anthraquinone-1-sul — fonic acid, the corresponding derivative of 1-aminoanthraquinone is formed. For example, methylamine yields methylaminoanthraquinone, which, like the non — methylated base, is an important starting material for valuable anthraquinone dyes.
The sulfo group in anthraquinone-1-sulfonic acid is replaceable, not only by amino and substituted amino groups, but still more easily by halogen. Thus, 1-chloroanthraquinone is formed in quantitative yield by treating the potassium sulfonate in boiling hydrochloric acid with sodium chlorate. The product is completely pure. The 2-sulfonic acid also undergoes this reaction, but much more slowly.
|
|
|
|
![]() СО
СО
m. p., 161 °С.
Reaction of the sulfonic acid with alcoholates or phenolates produces the ethers of erythrohydroxyanthraquinone (1-hydroxyanthraquinone):
![]()
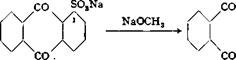 + Na2S03
+ Na2S03
23. 1,5- and 1,8-Dihydroxyanthraquinone, and
1,5-
 |
|
 |
|
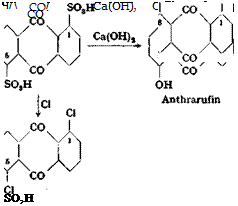
(a) Antkraquinone-lyS — and — l>8-di*ulfonic Acide80
An intimate mixture of 200 grams of pure anthraquinone (dried at 140°C.) and 4 grams of elutriated mercuric oxide is added with stirring, over a period of 30 minutes, to 400 grams of 18 per cent oleum,
80 Fierz-David and Krebser, Helv. Chim. Acta, 10, 200 (1927).
![]()
the temperature being raised simultaneously to 75-80°. The mixture is heated to 120° and stirred at this temperature for 1 hour, after which 140 grams of 66 per cent oleum is added dropwise over a period of 3 to 4 hours. Care is taken that no unreacted anthraquinone remains on the walls of the reaction vessel or on the stirrer. Sulfonation is complete after stirring for an additional 10 to 12 hours at 120°, as shown by the formation of a clear solution when a test portion is diluted with water. The reaction mixture is allowed to cool to 50° and is then diluted by slow addition, with stirring, of 100 grams of concentrated sulfuric acid. After standing overnight, the anthraquinone-l,5-disulfonic acid has precipitated completely in a pure state. The solid is filtered off on a sintered glass funnel, washed with 50 cc. concentrated sulfuric acid, and sucked as dry as possible. The filter cake is then dissolved in 2 liters hot water, and the resulting solution is filtered hot and treated with 100 grams of potassium chloride in 300 cc. hot water to precipitate the difficultly soluble potassium sulfonate. The mixture is tested for complete precipitation by filtering a test portion and adding more KC1 to the filtrate; no additional precipitate should be formed. The mixture is cooled, and the potassium salt is filtered off, washed with a total of 200 cc. cold water, and dried. The yield of potassium anthraquinone-1,5-disulfonate is 192 to 196 grams, or 45‘to 46 per cent of the theoretical amount.
The concentrated sulfuric acid filtrate from the 1,5-disulfonic acid is diluted with an equal volume of water which must be added slowly and with good stirring in order to prevent local overheating and a splitting off of sulfo groups. The diluted solution is allowed to stand for 2 and 3 hours to precipitate most of the 1,8-disul — fonic acid. This product is filtered off on a sintered glass funnel and washed with 44 per cent sulfuric acid. The free acid is converted to the potassium salt by dissolving it in 1.5 liters water and treating the hot solution with 80 grams of KC1 in 200 cc. water. The yield of potassium anthraquinone-1,8-disulfonate is 98 to 100 grams, or about 23 per cent of the theoretical amount.
The mother liquor from the 1,8-disulfonic acid contains about 30 per cent of the original anthraquinone in the form of a complicated mixture of disulfonic acids (mainly the 1,7 acid along with a small amount of the 1,6 isomer, still smaller amounts of the 1,5 and 1,8 acids, and traces of the 2,6 and 2,7 compounds). This mixture cannot be separated in any simple way. In the industry, the residue is worked up to produce "silver salt” by prolonged heating of the diluted sulfuric acid solution at 180-200°. This treatment splits out sulfo groups in the 1 position, and the resulting mixture of anthraquinone and anthraquinone-2-sulfonic acid is treated in the manner described under the preparation of sodium anthraquinone-2- sulfonate (page 228).
The 1,5- and 1,8-disulfonic acids can also be isolated as their sodium salts which are somewhat more soluble than the potassium salts. The reaction mixture is neutralized with soda in this case.
Frequently, the 1,5- and 1,8-disulfonic acids are not isolated separately, but are taken out of the reaction mixture together. For this purpose, the sulfonation mixture is first diluted with concentrated sulfuric acid, then with sufficient water to make the solution 60 per cent with respect to sulfuric acid.
(Ь) 1 гї — and 1,8-Dihydroxy anthraquinone
A mixture of 44.4 grams of dry, powdered potassium anthraquinone-
1,5- disulfonate (or the 1,8 isomer, or a mixture of the two), 700 cc. water, 46 grams of hydrate of lime — Ca(OH)2— and an aqueous solution of 5.5 grams of CaCl-,, is heated in an autoclave with stirring for 20 hours at 195-200°C. The pressure is 14 to 16 atmospheres. After cooling, the contents of the autoclave are transferred to a beaker and heated to boiling. Concentrated hydrochloric acid is added carefully until the mixture shows a strongly acid reaction to Congo red, and boiling is continued until the odor of S02 disappears. The greenish yellow precipitate is filtered off and washed with hot water until the washings are neutral. The yield is 20 to 21 grams, or about 85 per cent of the theoretical amount.
1,5-Dihydroxyanthraquinone (anthrarufin) melts at 280°C., the 1,8 isomer (chiysazin) at 191°. When the mixture of the 1,5- and 1,8-disulfonic acids is used, the resulting mixture of the two dihydroxy compounds has an indefinite melting point of 235-255°.
Both of the dihydroxyanthraquinones are important starting materials for alizarin and indanthrene dyes. In many cases, the two isomeric dihydroxyanthraquinones give very similar dyes, and a mixture of the two can be used directly (e. g., see alizarin saphirol В and SE).
(c) 1,5-Dichloroanthraquinone
A mixture of 82 grams of potassium anthraquinone- 1,5-disulfonate, 1 liter water, and 140 grams of concentrated hydrochloric acid is heated to 98-99°C., and a solution of 172 grams of potassium chlorate in 1500 cc. water is added, with stirring, over a period of 3 hours. The hot mixture is stirred until a filtered test portion gives, on cooling, no further precipitation of chloroanthraquinonesulfonic acid. This requires about 3 hours. The mixture is then filtered hot, and the product is washed with hot water until all of the acid is removed. The yield of dichloroanthraquinone, melting at 243-244°, is 45 grams, or about 88 per cent of the theoretical amount.
Both of the chlorine atoms are easily replaced by amino and phenoxy groups and the like, yielding technically important intermediates.
 11 ноября, 2015
11 ноября, 2015  Pokraskin
Pokraskin 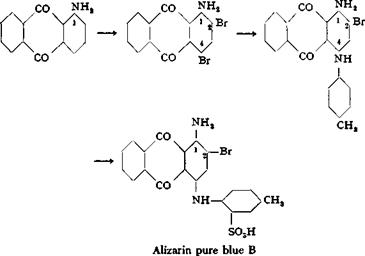
 Опубликовано в рубрике
Опубликовано в рубрике 