21. Anthraquinone Method It Oxidation of Anthracene73
|
CH CO
CH CO Anthracene Anthraquinone |
Impure anthracene should not be used in the preparation of anthraquinone, since too much chromic acid is required. The product now produced by coal tar distillation varies in purity from 80 to 92 per cent, the value for any given sample being determined by well known methods.74 The commercial product is always crystallized from pyridine bases.
Before oxidation, the anthracene must be sublimed with superheated steam at about 200°C. This is the only process which yields a product in a sufficiently high state of subdivision.
In a large lead-lined vessel, 300 grams of sublimed, still moist, anthracene (calculated on the basis of 100 per cent product) is stirred with 6 liters of water and 600 grams of sodium bichromate. The mixture is heated to 80°C. over a Fletcher burner, and 1800 grams of 50 per cent sulfuric acid is added over a period of 10 hours. Chromic — acid should always be present, and the mixture should be stirred with a wooden or glass rod. Finally, the mixture is boiled for 2 hours, replacing the water lost by evaporation. The precipitate is then filtered off and washed thoroughly. The mother liquor can be worked up to recover chrome alum or chromic sulfate.
73 See also, R. Gnehm, Die Anthracenfarbstoffe. Vieweg, Braunschweig, 1897.
74 Lunge and Berl, Chemische-technische Untersuchungsmethoden. 8th ed., Springer, Berlin, 1931.
The crude anthraquinone contains some unchanged anthracene and other impurities and must be purified before use. Purification is effected by partial sulfonation and distillation with superheated steam.
The pulverized, dry crude anthraquinone is heated at 120°C with
2.5 times its weight of 66° B6 sulfuric acid until no more sulfur dioxide is formed. This usually requires about 3 hours. The mixture is allowed to cool to 80°and enough water is added, over a period of 1 hour, to dilute the sulfuric acid to 30 per cent. This procedure causes the anthraquinone to separate in an easily filterable form, whereas if the sulfonation mixture is poured into water, a slimy precipitate is formed which is impossible to filter. The diluted mixture is cooled and filtered and the precipitate is washed thoroughly with water. The product is then sublimed with steam at 240-260°. (Apparatus, see Figs. 23 and 24). If it appears necessary, the purification operation can be repeated. In this case, the sulfuric acid should be only very slightly colored. The purified anthraquinone is obtained as a fine powder having a weak yellow green color. The yield from 100 grams of pure anthracene is about 106 grams of 100 per cent anthraquinone.
Technical Observations. The industrial oxidation of anthracene is carried out in lead-lined wooden or iron vessels of very large size. Vats of 15,000- to 25,000- liter capacity are not unusual. The chromic sulfate obtained as a by-product is an important consideration in the process, since it is used in large quantity in the chrome tanning of leather and cannot be produced as cheaply in any other way. Because of the demand for chromic sulfate, anthraquinone is still produced, in great measure, by chromic acid oxidation despite the fact that anthracene can be oxidized satisfactorily on an industrial scale by other reagents, such as nitrous oxides and air, or simply by air alone in the presence of a vanadium catalyst. The latter method is used today to produce a significant amount of anthraquinone. If chrome tanning is ever replaced by tanning with synthetic tannins, the chromic acid oxidation method will certainly disappear.
Distillation of anthracene and anthraquinone is carried out in apparatus quite similar to that used for diphenylamine (page 142). The steam is condensed in large chambers (about 3 x 3 x 5 m.), in which cold water is sprayed. The bottom of the chamber consists of a fine calico screen which retains the sublimate and allows the water to pass through.
|
Method 2: From Phthalic Anhydride and Benzene
|
(a) o-Benzoylbenxoic acid
In a 3-necked flask fitted with stirrer and thermometer are placed 148 grams (1.0 mole) of phthalic anhydride, which must be completely free from phthalic acid,* and 520 grams of thoroughly dried benzene. To this solution is added, in one portion, 267 grams (2 moles) of anhydrous aluminum chloride (this amount of A1C1:( is necessary to prevent the formation of diphenylphthalide) which is broken up into peasized lumps. A reflux condenser fitted with a calcium chloride tube is then attached, and the mixture is warmed slowly to 70°C. with continuous stirring and held at this temperature until no more hydrogen chloride is generated. The mixture is then poured with stirring (foam!) into a solution of 400 grams of soda ash in about 2.5 liters of water, and the excess benzene is removed by steam distillation. The mixture should now be alkaline, and if not, more soda is added. The precipitated aluminum hydroxide is filtered off from the hot solution and washed with hot water, then heated again with about 1 liter water, filtered, and again washed until a portion of the washings gives no precipitate on the addition of acid. The combined filtrate and washings are made strongly acid to Congo red with concentrated hydrochloric acid. After cooling, the precipitated benzoylbenzoic acid is filtered off, washed with water, and dried. The yield is 215 to 220 grams, or 95 to 97 per cent of the theoretical amount.
(b) Anthraquinone
The dried benzoylbenzoic acid, obtained above, is dissolved in 6 parts of 66° Вё sulfuric acid and heated for 1 to 2 hours at 150°C. The solution is cooled and poured into water, and the precipitated anthraquinone is filtered off and washed thoroughly with hot water. Any residual benzoylbenzoic acid is removed by washing the precipitate with soda solution. The product is then washed with water and dried. The yield is almost quantitative.
Д-Methyl- and Д-chloroanthraquinone are prepared in the same way by using toluene and chlorobenzene, respectively, instead of benzene in the foregoing procedure (see next preparation, and also page 58), When chlorobenzene is used, it is recommended that the aluminum chloride be added gradually at 40-50°C. with stirring, and the temperature then raised to 80° and held at this point until the evolution of hydrogen chloride ceases.75
* The phthalic anhydride should be tested by heating a sample in a test tube. If the material does not produce a clear melt without formation of gas, it must be dehydrated by heating it carefully in a porcelain dish until a perfectly clear, nonfoaming melt is formed. The melt is then cooled in a desiccator.
79 Murch (Nat. An. & Chem. Co.), U. S. Pat. 1,746,736 (1930) [C. A., 24, 1651 (1930)1.
1amino2-methylanthraquinone
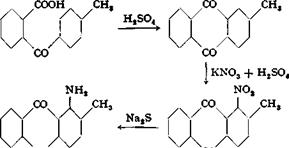
22, 1 -Amino-2-methylanthraquinone76
![]() сн,
сн,
со
J А1С13
|
The condensation of toluene with phthalic anhydride takes place in the presence ef aluminum chloride even at room temperature and is complete after about 12 to 15 hours of stirring. The ring closure can be effected with 5 per cent oleum (10 parts for 1 part of toluylbenzoic acid) by heating on a water bath for 2 hours. Usually, however, the preparation is done exactly as described for the preparation of anthra — quinone from phthalic anhydride and benzene. The yield of 2-methyl — anthraquinone, melting at 170-174°C., is about 85 to 88 per cent of the theoretical amount calculated on the phthalic anhydride.
 4 ноября, 2015
4 ноября, 2015  Pokraskin
Pokraskin 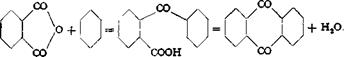
 Опубликовано в рубрике
Опубликовано в рубрике 