(the preparation starting with 1 mole would require too large a volume) of Д-naphthol is dissolved at 50°C. in a mixture of 65 grams of 35 per cent sodium hydroxide solution and 750 cc. water. To this solution, which should have a distinct, but weak, reaction to thiazole paper, are added 36 grams of 100 per cent sodium nitrite and enough water and ice to bring the volume to 1.5 liters and to lower the temperature to 0°. About 160 grams of 40 per cent sulfuric acid is then added over a period of three hours with good stirring. The solution should become distinctly acid to Congo red and show a positive reaction with starch- iodide paper. After at least 1 hour, the nitrosonaphthol is filtered off on a large suction funnel and washed thoroughly. It is chemically pure provided that pure /З-naphthol was used.
(a) Reduction and rearrangement to aminonaphtholsulfonic acid. The moist nitrosonaphthol is stirred with a small amount of water to form a uniform paste which is cooled in ice to 5°C., and 320 grams of sodium bisulfite solution (about 25 per cent S02) is added in one portion. (It is essential that the bisulfite solution be titrated to determine the S02 content. For each mole of /J-naphthol, 2.5 moles SO± are used.) The nitrosonaphthol goes into solution in a short time, but if necessary, a small amount of dilute sodium hydroxide is added carefully.
The solution is filtered to remove any tarry material which it contains. (The hydroxylaminesulfonic acid may be salted out to yield the Alsace green J or Dioxine N of the trade, a dye which is used to a certain extent in calico printing. Its iron, lake is very fast to light.)
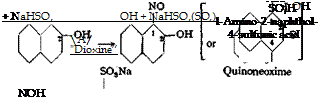 |
The filtered solution, about 1 liter in volume, is transferred to a beaker, and a mixture of 100 grams of 66° B6 sulfuric acid and 200 grams of water is added at 25°C. The solution should now have a strong mineral acid reaction. After 1 hour, the solution is heated to 50°, then allowed to stand overnight. The reaction mixture solidifies to a cake of the free aminonaphtholsulfonic acid, which is filtered off and washed thoroughly with water. The yield is about 90 per cent based on the /J-naphthol used.
The diazotization of l-amino-2’naphthol~4~sulfonic acid is described on pages 242 and 248; an alternate method for preparing the same diazo compound is given on page 178. Coupling with £-naphthol yields palatine chrome black 6B®2 (erio — chrome blue-black R, salicine black U), first discovered by Badische A. S.F., and independently and almost simultaneously by Geigy (Sandmeyer) and by Kalle (Elbel). Coupling with a-naphthol gives eriochrome blue-black B*:!, discovered by Geigy. Both of these dyes are very fast blue-black chrome dyes. It is interesting that the coupling reaction with a-naphthol in strongly alkaline solution involves only the position ortho to the hydroxyl group.
The diazo compound of l-amino-2-naphthol-4-sulfonic acid is so stable that it can be dried without danger, and can be nitrated in concentrated sulfuric acid solution with mixed acid.*1 The nitrated diazo compound reacts with the two naphthols to give the commercial chrome black wool dyes, eriochrome black T and A,65 which are cheap and almost unsurpassed for fastness.
The above method of sulfonation with sulfurous acid finds further application in the preparation of p-aminophenoldisulfonic acid from nitrosodimethylaniline and sodium bisulfite. In the rearrangement to the disulfonic acid, the dimethylamino group is split off with the formation of the p-aminophenol derivative. Pure dimethyl — amine is formed in the reaction.
«2 Badische A. und S. F., Ger. Pat. 156,440 (1904) [Frcfl., 8, 656 (1905-1907)1- «8 Geigy, Ger. Pat. 181,326 (1904) [Frdl., 8, 668 (1905-1907); C. A., 1, 2329 (1907)].
64 Sandmeyer and Hagenbach (Geigy), Ger. Pat. 164,655 (1905) [Frdl., 8, 647 (1905-1907)1.
«5Geigy, Ger. Pat. 169,683 (1906) [Frdl., 8, 673 (1905-1907)].
/З-Naphthylamine from /3-Naphthol
![]()
он jf Y Y-o—so, h
A mixture of 144 grams (1.0 mole) of pure /J-naphthol and 600 grams of ammonium sulfite solution is heated in an autoclave equipped with a stirrer and heated in an oil bath. (The ammonium sulfite solution can be prepared by saturating 250 grams of 20 per cent ammonia with S02, then mixing the resulting solution with an additional 250 grams of ammonia.) To this mixture is added 125 grams of 20 per cent ammonia, and the charge is heated 8 hours at a temperature (internal) of 150°C. and a pressure of about 6 atmospheres (steel tube manometer!). The autoclave is allowed to cool and the resulting cake of /J-naphthylamine is ground up in a mortar and washed thoroughly with water in a suction funnel. The ammonium sulfite solution can be used over again. The washed base is dissolved in a warm solution of 110 grams of hydrochloric acid (containing no sulfuric acid) and 1.5 liters water, and the solution is filtered to remove some unreacted /З-naphthol. The filtrate is mixed with a solution of 100 grams of anhydrous sodium sulfate in 250 cc. water to precipitate the /З-naphthylamine as the sulfate. The mixture is allowed to stand overnight, then the precipitate is filtered off and washed well with cold water. This product, after being dried, can be used directly for many purposes (see page 205).
To prepare the free base, the sulfate is mixed with 1 liter water and treated with 60 grams of soda ash dissolved in a small amount of water. The reaction requires several hours because of the low solubility of the sulfate, but it can be accelerated by continuous stirring and heating to 80°. The base is filtered off, washed, and dried at 80°. The yield is about 130 grams, or 85 to 95 per cent of the theoretical amount.
Technical Observations. It is absolutely necessary to use an autoclave heated’ by an oil bath or steam jacket for such reactions. The naphthylamine separates out at the bottom of the container as an oily layer, and unless an oil bath is used, overheating occurs to such an extent that, in spite of the stirring, a large part of the product is converted to dinaphthylamine and decomposition products. The same conditions prevail in the preparation of a-naphthol (page 180).
0-Naphthylamine is usually vacuum distilled in the plant. The distillation must be done very carefully, since the compound decomposes easily. For sulfonation reactions, if the free base is not isolated, the thoroughly dried sulfate, mixed with 1 per cent soda (see also primuline), is added to sulfuric acid or oleum.
The Bucherer method has completely replaced the older procedure of heating the naphthol with ammonia. This process gave only about a 70 per cent yield at pressures of 50 to 60 atmospheres. The Bucherer reaction was discussed in more detail on page 182.
 24 октября, 2015
24 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 