Nitrophenols are converted to their ethers by the general method outlined below.
One mole of the phenol is dissolved in 400 cc. water containing 1 mole of sodium hydroxide and 80 grams of soda ash. To this solution is added 500 cc. 90 per cent alcohol (ethyl or methyl) and the whole is cooled to 10°. Then 1.75 moles of ethyl or methyl chloride is added and the mixture is heated, with stirring or rbtating, in an autoclave at 100°C. and 4 to 5 atmospheres pressure for 8 hours. The alkylation is then completed. The mixture is poured into water, and the alkyl ether is separated, recovering the alcohol by rectification. The product, which is washed with small amounts of sodium hydroxide and water, should now contain no nitrophenol.
The following mixture can also be used advantageously, especially for nitro- cresol: 1 mole “phenol,” 600 cc. alcohol, 1.8 moles NaOH, 1.7 moles alkyl chloride, no water or soda.
Working with ethyl and methyl chlorides is not easy, and therefore the most suitable techniques should be described briefly. The mixture of nitrophenol, soda, and hydroxide in aqueous alcohol is placed in the autoclave and the latter is closed. It is not necessary that all of the material is dissolved. The autoclave is then evacuated With a water pump and the valve is closed. A small alkyl chloride tube, consisting of a tube
(1)
 |
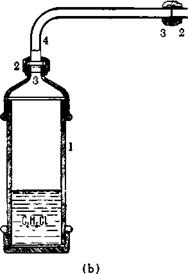 |
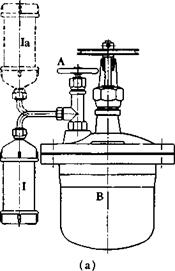
of 2-inch diameter with a screw top (3), is then connected to the autoclave through a copper tube (4) and packing nut (2) (Fig. 25b). The alkyl chloride has previously been poured into the tube, surrounded by ice water, from a cold graduated cylinder. (Methyl chloride is dissolved in an equal weight of cold methyl alcohol, and this solution is placed in the tube.) When the tube is attached to the autoclave, it is inverted (la) so that the alkyl chloride runs into the connecting tube.
Fig. 25. (a) Method of introducing alkyl chloride into a laboratory autoclave,
(b) Auxiliary cylinder (gas pipe) for handling alkyl chlorides.
This connecting tube is warmed with a hot wet towel until it is hot to the touch, and then the valve of the autoclave (A) is opened (Fig. 25a). The hot alkyl chloride is drawn into the autoclave by the vacuum, and the valve is closed after a few seconds. Tests have shown that at least 98 per cent of the alkyl chloride enters the autoclave under these conditions. The alkyl chloride tube, which is not now under pressure, is removed and the autoclave is heated in an oil bath as described above.
In the plant, alkylations are carried out in large horizontal or upright kettles (see chrysophenine). The alkyl chlorides are prepared from hydrochloric acid, zinc chloride (ferric chloride can be used equally well in place of zinc chloride) and alcohol, and are transported in large iron cylinders and stored in reservoirs. For use, the material is trans
ferred to weighed steel flasks, and pumped or driven by heat from these into the reaction vessel.
The alkyl ethers of o — and p-nitrophenols can also be prepared from o- and p-nitrochlorobenzenes bv the action of sodium alcoholates (see page 97).
 5 октября, 2015
5 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 