o — and p-Chlor о phenol from Phenol
|
OH OH OH
Cl |
The two isomers are prepared by the method of Dubois (1866), by the action of excess sulfuryl chloride on phenol, and are separated by fractional distillation.
In a 1-liter three-necked flask fitted with a stirrer, thermometer, dropping funnel, and gas exit tube, is placed 380 grams (4 moles) of phenol, and 610 grams (4.5 moles) of sulfuryl chloride is added dropwise over a period of 12 hours while the temperature is maintained at 20-25°C. Stirring is continued for an additional 5 hours at 10°, and then air is blown through the mixture for another 12 hours.
The reaction mixture is then distilled in vacuum through a fractionating column. At a pressure of 20 mm., the o-chlorophenol goes over at 75-90°, the para isomer at 110-115°. About 130 grams of o-chlorophenol (25 per cent) and 320 grams of p-chlorophenol (62 per cent) are obtained.
Remarks. The action of free chlorine on phenol is so vigorous that trichloro — phenol is formed at once. Monochlorophenol can also be prepared by adding the calculated quantity of sodium hypochlorite solution to a cold solution of phenol in sodium hydroxide; the product obtained by this method, however, is chiefly o-chlorophenol.
The chlorophenols have a disagreeable, clinging odor and must therefore be handled carefulfy.
Chloranil[30]
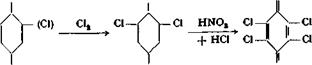 |
(Cl) Cl о
A 2-liter three-necked flask is fitted with a gastight stirrer (sealing liquid, either concentrated sulfuric acid or paraffin oil), gas introduction tube, and reflux condenser connected to a hood through a glass tube. In the flask are placed 47 grains (0.5 mole) of phenol, or 65 grams of o~ or p-chlorophenol, and 1 liter of technical concentrated hydrochloric acid (21° Be). Stirring is started, sufficiently vigorously to break up the mixture into very fine droplets, and a rather rapid stream of chlorine is introduced. The chlorine need not be specially dried, but it should be run through a wash bottle of sulfuric acid to show the velocity of the gas stream. The temperature rises to about 40°C. After 4 hours, the reaction flask is surrounded by a 70° water bath, and the introduction of chlorine is continued until the solution is completely saturated, requiring about 20 hours. As the chlorination proceeds, some crystals form on the upper part of the flask and in the condenser. When no more chlorine is being absorbed, the gas introduction tube is replaced by a dropping funnel, and 250 cc. nitric acid (40° Вё) is added over a period of 3 hours, while stirring is continued and the temperature is held at 80-85°. At first, a vigorous reaction takes place, but this soon subsides. The reaction mixture becomes red and the crystals dissolve. After the nitric acid has all been added, stirring is continued for 20 hours at a temperature of 85°. Heavy, plate-like, yellow crystals separate gradually. These are filtered off, after cooling, and washed first with 2 liters of water, then with 250 cc. alcohol to remove a reddish oily impurity. The product is then dried at 80°. It melts at 285-286° and is practically pure chloranil. The yield is 70 to 75 grams, or almost 60 per cent of the theoretical amount.
Remarks. All chloro derivatives of phenol are converted to chloranil by the above procedure. The process, therefore, offers a possibility for using the byproducts of phenol chlorination (page 145) which are often difficult to dispose of because of their poisonous nature and offensive odor.
Two of the chlorine atoms (para to each other) in chloranil are very reactive
and are replaceable by various other groups. Thus, reaction with aniline yields 2,5~dianilino-3,6-dichloroquinone:
О
which in itself has the properties of a vat dye for wool, and which is converted by sulfiding into a strong, very stable vat dye.[31] Chloranil reacts very easily also with sulfur derivatives, such as sodium sulfide, thiosulfate, potassium rhodinate, and others, and the sulfur-containing compounds thus obtained condense with the thiosulfonic acid of dimethyl-p-phenylenediamine, for example, to form blue sulfur dyes[32] (cf. methylene blue, page 311). This reaction is especially interesting in that it takes place stoichiometrically in the cold, just as in the formation of azo dyes.
It is also to be noted that chloranil is often very useful in the laboratory as a mild oxidizing agent, for example, in converting a leuco compound of the tri- phenylmethane series into the corresponding dye. In this reaction, the chloranil can be used in excess since it does not destaw the dye. Chloranil is too expensive to be used technically for such purposes (cl. page 401).
 |
 |
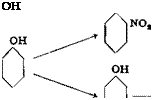
o — and p-Nitrophenols and Their Ethers
NO, NO,
A mixture of 94 grams of melted phenol and 20 cc. water is added dropwise to a solution of 150 grams of sodium nitrate in 400 cc. water and 250 grams of concentrated sulfuric acid. Good stirring is maintained during the addition, and the temperature is kept below 20°C. Stirring is continued for 2 hours. The mother liquor is poured off from the tarry mixture of nitrophenols, and the tar is melted with 500 cc. water with the addition of enough chalk to make the mixture neutral to litmus. The
wash water is poured off and the washing repeated. The crude nitro — phenols, freed from nitric acid, are subjected to steam distillation, using a condenser with a wide tube. About 40 grams of pure o-nitrophenol distills over. The residue in the distillation flask is cooled and filtered after standing for 24 hours. The precipitate is boiled with 1 liter of 2 per cent hydrochloric acid and filtered through a fluted filter. The pure p-nitrophenol crystallizes from the filtrate in nearly white, long needles. The extraction can be repeated if necessary.
The yield is about 40 grams each of the ortho and para isomers. It is bad practice to treat the crude nitrophenols with caustic soda, as called for in some procedures, because the caustic has an immediate resinifying action.
Technical Observations. In large scale operations, the distillation is carried out using either coil condensers, surrounded by warm water to prevent stoppage, or ordinary condensers supplied with warm water.
o — ana p-Nitrophenols are the starting materials for o — and p-phenetidine and amsidine. o-Nitroanisole is used in preparing dianisidine which gives the best direct blues in the trade (diamine pure blue, Chicago blue, and many others).
 3 октября, 2015
3 октября, 2015  Pokraskin
Pokraskin 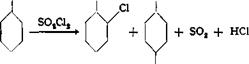
 Опубликовано в рубрике
Опубликовано в рубрике 