Just as* with aniline, many other aromatic amines (e. g., toluidine, xylidine, chloroaniline, a-naphthylamine, benzidine, aminoanthraqumone, and others) are converted into sulfonic acids by the so-called baking process as described above.[25] In contrast to the usual sulfonation procedures which use an excess of sulfuric acid or oleum, this process uses only the theoretical quantity of sulfuric acid and, as a result, the formation of higher sulfonation products is excluded. Isomeric byproducts are not formed, as a rule. The sulfo group almost always enters the position para to the amino group, or if this position is occupied, it enters the ortho position. The sulfo group never goes into the meta position, nor does it enter another ring if one is present. It is generally assumed that the reaction involves a preliminary formation of a sulfamic acid by splitting out of water, followed by a rearrangement to the aminosulfonic acid. This explanation cannot be correct, however, because tertiary amines (e. g., dimethylanjline) are also sulfonated in the baking process, even though the formation of intermediate sulfamic acids is not possible.
In order to achieve a smooth reaction, it is important that the acid sulfate be homogeneous and not contain local excesses and deficiencies of sulfuric acid. This is not easy to attain in practice, because clumping tends to occur when the acid and base are mixed, and thorough mixing is very difficult. It is often of help to use diluted sulfuric acid and, in the case of solid amines, to use a solvent for the base
(see the preparation of naphthionic acid, page 180); in these cases, the water must be driven on completely at a temperature below that of the reaction. In order to have uniform heating of the reaction mixture during the baking, the mixture should be spread out in a thin layer.
The optimum temperature lies between 170 and 220°C.; it varies with different compounds and must be established by experiment for each individual case.
It is highly advantageous to carry out the reaction under vacuum. In this way, the danger of carbonizing is avoided and the sulfonation proceeds more smoothly and rapidly. For these reasons, modern plants use vacuum baking ovens almost exclusively, heated either directly by name, or, better, by superheated steam. Electrical heating can also be used, having the advantage of easy controllability. A vacuum apparatus suitable for laboratory use is described on page 181 (Fig. 30).
1- (p-Sulfophenyl) -3-methyl-5-pyrazolone from Sulfanilic Acid
(a)
Phenylhydrazine-p-tulfonie Acid
A quantity of technical sulfanilic acid corresponding to 104 grams (0.6 mole) of 100 per cent material is dissolved in 400 cc. hot water containing 33 grams of soda ash. The solution is filtered and cooled, and 70 grams of concentrated sulfuric acid is added slowly with stirring. The solution is then cooled in an ice bath, and a solution of 42 grams of sodium nitrite in 100 cc. water is added dropwise over a period of 15 minutes, keeping the temperature below 12°C. Stirring is continued for another 15 minutes, after which the solution should show a strong acid reaction to Congo red and give a faint blue coloration on starch-iodide paper. If the latter is not the case, a few drops more of nitrite solution are added. The crystalline diazonium compound is filtered off with suction and washed with a small amount of cold water to remove the excess sulfuric acid.
The still moist (dry diazosulfanilic acid may explode violently!) diazonium compound is added slowly to a vigorously stirred solution of 340 grams of crystalline sodium sulfite (Na2S03 • 7H20) in 500 cc. water
![]()
|
(b) p-Sulfophenyl-3-methyI-5-pyrazolone CH.—co4 ,______ SO, H |4 в N SO3H / — Із 2/ ___ / * CH3-C-r—N + H20 + C2H5OH A solution is made of 18.8 grains (0.1 mole) of phenylhydrazine-p-sulfonic acid in 50 cc. hot water containing 6 grains of soda ash. The solution is filtered hot, and the small excess of soda is neutralized with dilute hydrochloric acid, making the solution neutral to litmus. After cooling the solution, 13 grams of acetoacetic ester is added slowly with stirring, and the solution is heated to 100°C. and stirred for 45 minutes at this temperature. The mixture is then cooled with stirring and acidified with 18 cc. concentrated hydrochloric acid. The precipitated pyrazolone is filtered off with suction, washed with cold water, and dried. The yield of slightly yellow powder is 24 grams, or 94 per cent of the theoretical amount. Technical Observations. Phenylmethylpyrazolonesulfonic acid is used, for example, in the preparation of fast light yellow G (page 265). Phenylmethylpyra — zolone itself (from phenylhydrazine and acetoacetic ester) and many of its derivatives are valuable components for the preparation of yellow azo eyes which, for the most part, are characterized by very high stability. Pbenylmethylpyrazolone is also a very important starting material for certain pharmaceutical products (antipyrine, pyramidone, etc.). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
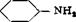
![]()
![]()
(a) Acetanilide from Aniline
NH, + CHjCOOH __ ^>— NHCOCH, + HsO
The reaction is carried out in a 500-cc. round-bottomed flask, heated by an oil bath, and fitted with a fractionating column, preferably of the type described on page 342. The column should be at least 20 cm. long and filled with glass rings; it should be insulated and equipped with a partial condenser cooled by a stream of air.
In the reaction flask are placed 186 grams (2 moles) of aniline and 180 grams (3 moles) of glacial acetic acid, a boiling chip is added (the mixture tends to bump badly), and the mixture is heated to gentle boiling (oil bath temperature 150-160°C.). As soon as the column has become warm and the vapor has reached the partial condenser, a rather strong stream of air is passed through the latter. Most of the vapor condenses and returns to the column, and only a very slow distillation takes place, the temperature being 102° at the most. After about 1 hour, the distillation almost stops, and the temperature of the oil bath is raised to 190° during the course of the next hour. It is held at this temperature until distillation at a temperature of 102° again ceases. At this point, 45 to 50 grams of distillate has been collected, consisting of 30 to 40 per cent acetic acid. Another 60 grams of glacial acetic acid is now added to the reaction mixture, and the distillation is continued for about 1 hour more, the distilling temperature staying between 100 and 102° with an oil bath temperature of 180-190°. The acetylation, which is now nearly completed, is finished by raising the oil bath temperature slowly to 220°; the distillation temperature increases to about 110° as the distillate becomes poorer in water. The heating is continued until a test of the reaction mixture shows the absence of aniline. This test is made by removing a drop of the mixture on a glass rod, mixing it with a little ice and dilute hydrochloric acid, and adding a few drops of nitrite solution. If aniline is present, benzene diazonium chloride is formed, which gives an intense orange red color when the solution is added to an R salt solution containing soda. If the test for aniline is negative, the oil bath is allowed to cool to 180°, the column is removed, and the remainder of the acetic acid is distilled out in vacuum at an oil bath temperature of 180°. For this operation it is desirable to install a capillary tube to draw air through the mixture. The residue is poured, while still hot, into a porcelain dish or onto a copper plate, where it solidifies to a crystalline mass. The product is colorless if the aniline used was colorless. The material melts at 114-
115°. The yield is at least 265 grams, or 98 per cent of the theoretical amount.
The crude material can be used without further treatment for the following nitration step. Especially pure acetanilide, such as is required for pharmaceutical purposes, can be obtained by recrystallization from boiling water, yielding completely colorless and odorless, shiny plates.
(b)
 |
p-Nitroacetanilide from Acetanilide
In a tightly covered iron beaker (see Fig. 19) or a three-necked flask, fitted with thermometer and dropping funnel, a mixture is made with stirring, of 135 grams (1.0 mole) of finely powdered, dry acetanilide and 540 grams of concentrated sulfuric acid (66° Be). The temperature should not rise above 25°, or hydrolysis of the acetanilide may occur. The acetanilide dissolves completely in 1 to 2 hours. The clear solution is cooled in an ice-salt mixture (in order to obtain an efficient freezing mixture, it is absolutely necessary to weigh out the constituents—3 parts of finely divided ice and 1 part of salt—and mix them thoroughly) and a mixture of 105 grams of 62 per cent nitric acid {40° Вё) with 105 grams of sulfuric acid (66° Вё) is added from the dropping funnel over a period of about 1 hour. The reaction temperature should not exceed 2- 3°; preferably, it should be kept even lower, down to about —5°, since the lower the temperature, the less of the o-nitro compound is formed. Under the right conditions, such as prevail in industrial operations, the theoretical amount of nitric acid can be used. A small excess does no harm, however, because the entrance of a second nitro group into the molecule takes place only with some difficulty. Stirring at 0° is continued for about 3 hours after the mixing is completed, and then a nitrometer test (page 72) is made to see whether the nitric acid remaining corresponds to the small excess used. An additional test is also made by mixing a small test sample with water, adding sodium hydroxide, and boiling. No odor of aniline should be noticeable.
The reaction mixture is poured, with good stirring, into a mixture of 350 cc. water and 350 grams of ice. The nitroacetanilide precipitates im-
mediately, and after an hour it can be filtered off without loss. It is washed thoroughly with water, then slurried with 700 cc. water, enough soda is added to make the mixture distinctly alkaline to litmus, and the mixture is heated to boiling. This treatment hyrolyzes only the o-nitro — acetanilide. The residue is filtered off at 50° and washed thoroughly with water, yielding about 90 per cent of the theoretical amount of p-nitroacetanilide.
The acetyl derivative can be hydrolyzed with sodium hydroxide. The moist press cake of p-nitroacetanilide is mixed with an equal weight of water, 200 grams of 35 per cent sodium hydroxide is added, and the mixture is heated to boiling. This treatment hydrolyzes only the o-nitro — 3 hours, a test sample should dissolve to form a clear solution in 15 per cent hydrochloric acid, showing complete hydrolysis. The mixture is cooled to 40° and filtered. The product, washed carefully with cold water, is chemically pure. The yield for 93 grams of aniline is about 100 grams of p-nitroaniline.
 26 сентября, 2015
26 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 