15.6 grams (0.1 mole) of m-chlorodimethylaniline* is dissolved in 40 cc. concentrated hydrochloric acid, and after cooling, 7.7 cc. 39 per cent formaldehyde is added. This mixture is poured into the well stirred solution of 0.125 mole of p-tolylhydroxylaminesulfonic acid, i. e., 225 cc. of the solution prepared above. Then, 70 cc. of a 20 per cent ferrous sulfate solution is added immediately. The reaction mixture warms up to about 40°C., and after a few minutes crystals begin to separate, the whole mixture soon becoming a thick paste which is made more easily filterable by stirring. After 12 hours, the benzylidene compound is filtered off with suction and washed with 20 per cent salt solution. The filtrate is made alkaline with sodium hydroxide and steam distilled to recover 3.6 grams of chlorodimethylaniline.
* Obtained from m-chloroaniline by the procedure given for dimethylaniline, page 133. m-Chloroaniline is prepared from m-nitrochlorobenzene, page 116, exactly as aniline is prepared from nitrobenzene, page 75. m-Chloroaniline is very poisonous.
For the hydrolysis, the benzylidene compound is dissolved in 300 cc of a warm 2 per cent soda ash solution, and the solution is filtered to remove a residue of iron carbonate. The solution is then treated with 5 cc. concentrated sodium hydroxide solution (40° Вё) and heated with stirring for 1 hour on a water bath. The solution becomes cloudy and the aldehyde separates in oily drops which solidify on cooling. It is dissolved in 1:1 hydrochloric acid and reprecipitated with 2 N soda solution. The yield is 13.2 grams of dry aldehyde melting at 81° (72 per cent calculated on die base used, or 94 per cent calculated on the unrecovered base). The aldehyde can be recrystallized from ligroin. It is used, for example, in the preparation of wool blue 5B (see page 305).
|
m-Nitrobenzenesulfonic Acid and Metanilic Acid from Nitrobenzene
Sulfone |
In a cast iron kettle, 123 grams (1.0 mole) of nitrobenzene is added carefully at 70°C. to a threefold quantity of oleum containing 25 per cent S03. The mixture heats up rapidly to 100-110° but should not be allowed to go higher or sudden charring may occur. When the addition of nitrobenzene is completed, the mixture is heated at 110-115° until a test sample in water has no trace of nitrobenzene odor. If the sulfonation is not complete in 30 minutes after the mixing, the S03 is insufficient and 50 grams more of oleum is added dropwise. If necessary, another 50-gram portion is added after another half-hour. If the oleum actually contains 25 per cent of S03, however, these additional quantities should not be necessary. When the sulfonation is completed, the reaction mixture is cooled and poured onto 500 grams of ice with good mechanical stirring. The nitrobenzenesulfonic acid goes into solution, leaving a small amount of sulfone undissolved.
The acid can be worked up by various methods, such as, for example, that given for benzenesulfonic acid on page 80 ff. It is preferable, however, to salt out the sulfonic acid since its sodium salt is practically insoluble in saturated salt solution. With continuous mechanical stirring, 200 grams of salt is added in small portions. Sodium nitrobenzenesul- fonate separates, forming a thick paste which gradually becomes more fluid on long continued stirring. After about 10 hours, the solid is filtered off on a large suction funnel and pressed out for several hours in a cotton cloth in the screw press. The sodium salt is usable technically without further purification. It can be obtained pure by recrystallizing from water.
|
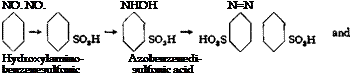
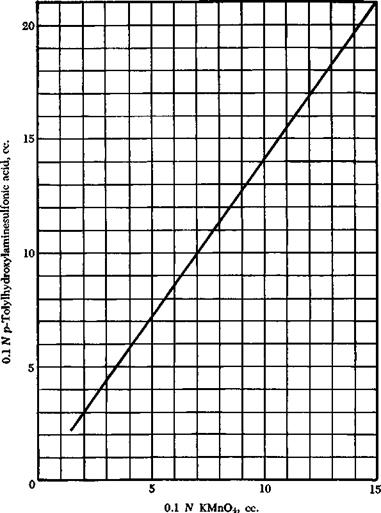
Fig. 22. Curve for determination of p-tolylhydroxylaminesulfonic acid. |
The reduction of m-nitrobenzenesulfonic acid is carried out by the method given for preparing aniline (page 75), except that the iron need not be etched since the free mineral acid in the press cake is sufficient to start the reaction. In an iron or copper reduction beaker of about 2-liter capacity are placed 250 grams of finely pulverized cast iron and 1 liter of water. This mixture is heated to boiling over a free flame and then, with brisk stirring and continued heating, the broken-up press cake is added in small portions during the course of 1 hour. The evaporated water is replaced from time to time so that the volume is held at about 1 liter. Boiling is continued for another 20 minutes and a spot test on filter paper is made to determine whether the solution is nearly colorless; it should be only very light brown in color, never dark brown or deep yellow. If the solution is decolorized, soda ash is added, very carefully to avoid foaming over, until the solution is strongly alkaline to litmus and a spot test on filter paper gives no blackening with sodium sulfide solution. The iron sludge is then filtered off with suction and washed well with hot water. By evaporating the filtrate to 600 cc. and making it acid to Congo red with hydrochloric acid, the metanilic acid is precipitated in the form of fine crystals. Many plants prefer to use the concentrated solution directly, since metanilic acid is quite soluble and its separation always entails a 10 to 15 per cent loss. This loss is offset, however, by higher dye yields. The yield is determined simply by titrating the mineral acid solution with sodium nitrite; it amounts to about 90 per cent, or 155 grams of the pure acid.
Remarks. Ferrous sulfate can be used advantageously instead of salt to precipitate the m-nitrobenzenesulfonic acid. The iron salt is very difficultly soluble in 20 per cent sulfuric acid and can be filtered off directly without further treatment. The reduction is carried out exactly as described above. If benzene is sulfonated with 100 per cent sulfuric acid, and then the benzenesulfonic acid is nitrated at 100°, there are formed, according to the work of Obermiller,[20] [21] [22] [23] [24] besides the meta sulfonic acid, considerable quantities of the ortho and para isomers. The latter can be separated quite easily through the magnesium salts, and this affords a method for obtaining the valuable o-aminobenzenesulfonic acid. The preparation of orthanilic acid starting with o-nitrochlorobenzene has also been described.97
It is to be observed that other m-nitrosulfonic acids also form very insoluble ferrous salts. One example is 2-nitro-4,8-naphthalenedisulfonic acid which can be obtained in a pure state very easily by this method.38 This method of separation has long been known in the industry. For information about 2-naphthylamine-4,8- disulfonic acid, see page 219 ff.
Analogous Sulfonations. Exactly the same method can be used for sulfonating p-nitrochlorobenzene, p nitrotoluene, o-nitrochlorobenzene, chlorobenzene, ana many other compounds. On the other hand, it is usually not possible to sulfonate dinitro compounds in this way. Dinitrochlorobenzene and dinitrotoluene are decomposed explosively by treatment with fuming sulfuric acid. If dinitrochloro — benzenesulfonic acid is to be prepared, for example, one starts with p nitrochloro — benzene» This is sulfonated, as described previously, and the sulfonic acid is converted to dinitrochlorobenzenesulfonic acid by treatment with mixed acid (50:50 sulfuric and nitric acids) at low temperatures. This product yields, on replacement of the chlorine by —OH and partial reduction, 4-nitro-2-aminophenol-6-sulfonic acid (nitro acid III), which is used in preparing chrome dyes.
Dinitronaphthalenes are converted to naphthazarin by oleum.39
Technical Observations. Sulfonations of this type are carried out on a large scale in kettles provided with jackets through which either steam or cooling water can be circulated as desired. The reaction mixtures often become quite hot, and the operations must be conducted carefully so that dangerous overheating and explosion are avoided. Salting out is done in wooden vats, and the precipitate
is pressed out in filter presses exerting 250 atmospheres pressure. Reduction, evaporation, and working up of the reaction products, are carried out as described previously.
2,2
 |
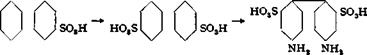
-Benzidinedisulfonic Acid from Nitrobenzene
|
|
|
О acid II |
|
N=N NH—- NH |
|
Azoxybenzene — Hydrazobezene* 2,2′-Benzidine- disulfonic acid disulfonic acid disulfonic acid |
Nitrobenzenesulfonic acid is prepared as described under metanilic acid. The reduction is different from similar reactions only in that it is carried out in dilute aqueous solution in three distinct steps, making it possible to obtain the benzidinedisulfonic acid with-a minimum amount of zinc dust and sodium hydroxide.
If the sodium salt is not entirely pure, the press cake of sodium nitro — benzenesulfonate, from 100 grams of nitrobenzene, is dissolved in water and about 30 grams of soda ash is added to make the solution exactly neutral to litmus. The solution is made up to 1.5 liters and cooled to 10°C. and 10 grams of ammonium chloride is added. While the solution is stirred vigorously, 120 grams of zinc dust is added portionwise over a period of 2 minutes. The temperature is kept below 20° by the addition of finely shaved ice, and stirring is continued for 20 minutes. Then 120 grams of 30 per cent sodium hydroxide is added in one portion and the solution is warmed to 70° without stirring. The solution, which was colorless, immediately turns orange yellow as a result of the formation of azo- and azoxybenzenedisulfonic acids. It is now allowed to stand at least 3 hours, or better, overnight.
The solution is then carefully neutralized by the dropwise addition of about 90 grams of concentrated hydrochloric acid to the point where the reaction to thiazole paper disappears. It is then heated to 80°, and 40 grams of zinc dust is added. If the solution is not decolorized in 5 minutes, more hydrochloric acid is dropped in slowly, keeping the tempera
ture at 75-80°. The solution is decolorized, i. e., is changed from a dirty brown to a light gray, in less than 5 seconds after the neutral point has been reached. The solution, whose volume should be about 1.8 liters, now contains the hydrazobenzenedisulfonic acid. It is filtered immediately in order to prevent further reduction to metanilic acid, washing the zinc dust well. The filtrate is cooled to 20°, and 120 grams of concentrated hydrochloric acid is added. After a few minutes, a shiny precipitate of colorless, hard crystals of 2,2′-benzidinedisulfonic acid begins to form, and the solution turns yellow due to partial disproportionation yielding azobenzenedisulfonic acid and metanilic acid. A few grams of zinc dust is added to decolorize the solution. Although benzidinedisulfonic acid is extremely insoluble in water (1 liter of water dissolves less than 1 gram), the product separates very slowly. It should be allowed to stand for 2 days, then the precipitate is filtered off and washed with cold water. The yield is about 65 grams.
Technical Observations. In large scale operations, involving volumes of 4000 to 5000 liters, the crystallization of 2,2′-benzidinedisulfonic acid requires at least 3 days. In order to obtain rapid cooling, lead coils, through which cold water is circulated, are installed in the wooden vats. The sulfonic acid must be diazotized indirectly because of its insolubility. It is dissolved in the required amount of water and soda, the neutral solution is mixed with sodium nitrite, and the mixture is allowed to flow in a thin stream into hydrochloric or sulfuric acid.
As with all benzidine derivatives with substituents in the position ortho to the diphenyl bond, 2,2′-benzidinedisulfonic add gives no substantive cotton dyes, but, in general, yields acid wool dyes of exceptional stability to washing and milling (e. g., acid anthracene* red C, page 294). Its combination with 2 moles of salicylic acid is a valuable dye for chrome printing on cotton (chromocitronine, page 295).
Benzidine from Nitrobenzene
![]() (intermediate steps: azoxybenzene and
(intermediate steps: azoxybenzene and
azobenzene)
NH,(HC1)
со. 85%, Benzidine
In a glass flask equipped with an efficient stirrer and a reflux condenser is placed a mixture of 125 grams of nitrobenzene and 250 cc. o-di- chlorobenzene (solvent naphtha of boiling point about 170°C. can also
be used). To this mixture are added, alternately, zinc dust mixed with o — dichlorobenzene to form a thin paste, and 50 per cent sodium hydroxide solution. The nitrobenzene-o-dichlorobenzene mixture is first heated to 115-125°, then 5 cc. of the hydroxide solution is added, followed by 10 grams of the zinc dust paste. Reduction should take place rapidly, but, if not, the mixture is heated to 130°. Once the reaction has commenced, the alternate additions of the reagents are made at such a rate that the temperature remains at 115-120°. Care should be taken that some of the unreacted zinc dust does not settle to the bottom of the flask, or the reaction may suddenly become violent. The additions should be complete after about 3 hours. About 250 grams of the sodium hyroxide solution and about 260 grams of zinc dust are used. The reaction mixture first becomes red, then gradually decolorizes and turns white due to the sodium zincate hydrate. Stirring is continued until the mixture is completely decolorized, which may require 4 to 10 hours, depending on the manner in which the reaction is carried out and on the quality of the zinc dust used. If the reduction to hydrazobenzene is still incomplete after an even longer time, a few cubic centimeters of water is added carefully, and then, if necessary, a little more zinc dust and sodium hydroxide.
When the reduction is complete, water is added in small portions until the zinc sludge separates from the hydrazobenzene solution in a sharp layer. The o-dichlorobenzene layer is drawn off and the zinc sludge is washed twice with small portions of o-dichlorobenzene.
Rearrangement of Hydrazobenzene to Benzidine. The o-dichlorobenzene solution of hydrazobenzene is mixed with kn equal weight of finely pulverized ice to which 300 cc. concentrated hydrochloric acid has been added. The benzidine hydrochloride which is formed (along with the by-product, diphenyline) goes into the water layer. After 3 hours, the mixture is warmed to 80°, 500 cc. hot water is added, and the
o-dichlorobenzene layer is separated and washed twice with water. If the water layer is cloudy, it is filtered hot.
The solution of benzidine hydrochloride is mixed with good stirring with a solution of 100 grams of anhydrous sodium sulfate (or the corresponding amount of the hydrated salt). The relatively insoluble benzidine sulfate precipitates immediately, while the by-products remain in solution. After an hour, the sulfate is filtered off and washed well with water. [Note: if, in the rearrangement, the solution becomes red (formation of azobenzene), a few grams of iron shavings is added to give complete reduction.]
Preparation of the Free Base. The moist benzidine sulfate is stirred with a fivefold quantity of hot water and treated with enough solid soda
ash (about 45 grams) to give a definite and permanent alkaline reaction with litmus. The free base is filtered off and washed with water. It is obtained in the form of gray grains, which are dried at 100°. The product may be purified by distillation in vacuum (sausage flask). Its melting point is 128°. A yield of about 75 per cent of the theoretical amount is obtained, the remainder going into diphenyline and other by-products.
Technical Observations. The manufacture of benzidine is one of the most important operations of dye chemistry, because this base is used in the preparation of numerous valuable, although generally unstable, direct dyes. Similar methods are used to prepare the tolidines (ortho and meta) from o — and m-nitrotoluenes, and o-dianisidine from o-nitroanisole. Dyes from m-tolidine will rarely go on cotton, but they are interesting wool dyes.
The precipitate of zinc dust and zinc oxide is spontaneously inflammable and hence should not be discarded in the waste jar.
In addition to the method described, which is the one commonly used, the reduction can also be effected with iron shavings. This method, however, is difficult to carry out. There is also the electrolytic method in which nitrobenzene, in suspension m sodium hydroxide solution, is reduced to hydrazobenzene at the cathode. So far as is known, this method is used only by the GeseUschaft fiir Chemische Industrie in Basel. It does not require zinc dust and gives good yields. The best results are obtained when the specific gravity of the hydroxide solution equals that of nitrobenzene.
Distilled benzidine usually gives better results than the undistilled material, but it is somewhat more expensive. Only a little loss is entailed in the distillation, the residue being made up largely of impurities.
In the industrial preparation of benzidine derivatives, 200 to 500 kilograms of the nitro compound is reduced at one time — the operation requiring rather large apparatus. Less solvent can be used in these large scale operations than in the laboratory preparation because more efficient stirring can be used. The reduction requires about 24 hours. In the laboratory, the reduction, carried out according to the above probedure, succeeds only if the reaction mixture is thoroughly churned up. If this thorough mixing is not possible, as, for example, when working with very small quantities, better results are obtained if the o-dichlorobenzene is replaced by a water-miscible solvent such as alcohol.
8.
 |
Derivatives of Aniline Sulfanilic Acid from Aniline (Baking Process)
SO, H
In an iron dish, 105 grams (1.0 mole) of sulfuric acid (66° Вё) is poured, in a thin stream with good stirring, into 93 grams (1.0 mole) of
aniline. Industrially, this operation is carried out in an iron kettle and the mixture is stirred by hand with an iron rod. The resulting hot, thick paste is spread out on an iron plate (15 X 15 cm.) having a rim about 2 cm. high. The layer should be about 1 cm. thick. In the plant, layers up to 8 cm. thick are used. The mass is placed in a drying oven, at least 5 cm. from the flame, and heated for 8 hours at an air temperature of 190°C. The cake is then removed from the oven and knocked from the plate. The light gray product consists of about 90 per cent of sulfanilic acid and about 3 per cent of unchanged aniline along with a small amount of carbon. This crude sulfanilic acid can be used directly for many purposes by dissolving it in a carbonate solution in order to make a solution strongly alkaline to litmus; in this preparation, 60 grams of soda ash in 500 cc. water is used. This solution is boiled, maintaining the volume by replacing the water distilled out, until all of the easily volatile aniline has been steamed out. The remaining solution, after being filtered through cotton, meets the requirements for most industrial uses. In order to obtain pure sulfanilic acid, the solution is made strongly acid to Congo red with sulfuric acid, whereupon the sulfanilic acid precipitates in high purity, although it is still not sufficiently pure for analytical purposes (see Analytical Section). The yield of crude material is about 175 grams; after purification by precipitation, the product weighs about 140 grams.
 26 сентября, 2015
26 сентября, 2015  Pokraskin
Pokraskin 
 Опубликовано в рубрике
Опубликовано в рубрике 