The common reduction methods, using iron in acid or almost neutral solution, are not usable for selectively reducing one of several nitro groups present in a molecule. This is true also for those cases where some other easily reducible group, such as the azo group, is present along with the nitro group (see page 289). Iron attacks all the reducible groups simultaneously. For these "partial reductions,” sodium sulfide or some other sulfide is generally used technically. In many cases, even these reducing agents must be used with care, in order to restrict the reduction, adding the reagent gradually, avoiding an excess of it, and operating at the lowest temperature at which the reaction will proceed. The optimum temperature differs with individual cases; it may vary from 0° to 100°C. and must be determined, by tests, along with the other reaction conditions, in each instance. The reactions are usually carried out in water solution or, if necessary, in aqueous alcoholic solution.
With dinitro compounds in which the nitro groups are in the 2 and 4 positions relative to an alkyl, hydroxyl, alkoxy, or amino group, partial reduction usually leads to reduction of the 2-nitro group.
The sodium sulfide is converted in the reaction mainly into sodium thiosulfate according to the reaction:
4 X—NO, + 6 Na, S + ^ H,0 = 4 X—-NH, + 6 NaOH + 3 Na, S,0,
Hence, about 1.5 moles of Na2S is needed for each nitro group to be reduced. (The reduction does not always follow exactly the reaction given. Hence, the optimum quantity of sodium sulfide may vary somewhat from the theoretical amount and must be determined by trial.) Frequently, the hydroxide formed in the reaction has a deleterious effect, and in this event better results are obtained with sodium hydrosulfide (NaSH), i. e., a solution of sodium sulfide saturated with hydrogen sulfide. The reaction then proceeds without forming hydroxide, according to the equation:
4 X—NO, + 6 NaSH + H,0 = 4 X—NH, + 3 Na, S,0,
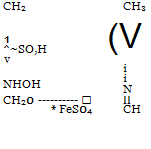
If the substance being reduced contains an acid group, this may serve to neutralize the alkali formed (see, e. g., the preparation of picramic acid, page 152). It may also be beneficial to add MeS04 to the reduction mixture. Since this compound reacts with the NaOH formed to precipitate very slightly soluble Mg(OH)2, the solution can become only weakly alkaline.
Certain nitro compounds must be reduced in ammoniacal solution with exactly the calculated amount of hydrogen sulfide. Thus, dinitrophenol can be reduced successfully to nitroaminophenol only by treating the very finely divided sodium salt, directly as it is obtained by the hydrolysis of dinitrochlorobenzene, with exactly the calculated quantity of hydrogen sulfide at about 60° (see sulfur black T). The nitroaminophenol obtained in this way is best recrystallized from boiling water. The difficultly soluble diazonium compound from nitroaminophenol coupled with in- phenylenediaminesulfonic acid (page 103) gives the cheapest chrome brown for wool. The coupling reaction is carried out in neutral solution, as concentrated as possible, for 2 to 3 days at 28°.
|
OH NH,
NO, SO, H Chrome brown R (Kalle) |
m-Phenylenediamine from m-Dinitrobenzene
In the reduction vessel (cf. page 75 and Fig. 17) are placed 1500 cc. water and 400 grams of fine iron shavings (page 75). The iron is etched with 20 cc. concentrated hydrochloric acid by boiling the mixture for at least 5 minutes. Then, with continuous stirring, 168 grams of pure dinitrobenzene is added in portions of not more than 2 grams each. The reaction mixture first turns yellow, due to the formation of m-nitroaniline, and each addition causes foaming which is often so violent that water must be squirted onto the surface of the reaction mixture. For satisfactory reduction, the temperature should be kept at the boiling point, and each new addition of dinitrobenzene must be delayed until a drop of the reaction mixture on filter paper is colorless. If the additions are made too rapidly, the mixture become brown due to the formation of azoxy compounds. If this happens, the reduction is unsuccessful and should be discontinued. The trouble may also arise from a bad batch of iron, emphasizing the need for testing each iron sample for its reactivity before purchasing it. Ordinarily, however, the dinitrobenzene can be reduced satisfactorily in 40 minutes, yielding a solution which is light brown in color, or often almost colorless and turning dark rapidly. Boiling is continued for at least 5 minutes, replacing the evaporated water
so as to maintain the original volume of about 2 liters. This solution contains about 45 grams of diamine per liter.
About 10 grams of solid soda ash is now added in small portions to the boiling solution to make it distinctly alkaline to litmus, and boiling is continued for 5 minutes to decompose the soluble iron complex of the hydroxylamine which is present. This boiling is continued until a test drop on filter paper gives no dark flecks when treated with sodium sulfide solution (1:10). The sodium sulfide test should not be omitted even in large scale reactions, since it can save much trouble. (If the test for iron does not disappear even after prolonged boiling, the residual iron is precipitated with a small amount of ammonium sulfide.) The solution is then filtered into a warm flask, and the filtrate is treated with the amount of hydrochloric acid necessary to produce a weakly acid reaction to litmus. The m-phenylenediamine solution prepared in this way is quite stable. The yield is about 95 grams. The solution can be analyzed with diazotized aniline in very dilute solution at 0°, or analogously with H acid except that no soda is added (see Analytical Section).
The technical solution obtained above is sufficiently pure for many purposes, but generally a purer product is desirable since it gives better yields of dyes. Purification is accomplished by evaporating the solution (not acidified), first over a free flame, then in vacuo, until the residue contains 40 per cent of the base. This concentrated solution can be completely evaporated in vacuum and the base distilled or, better, the base may be frozen out of the 40 per cent solution at 0°. In order to induce crystallization in this “cold process,” the solution must be seeded with a crystal of the phenylenediamine. The pure diamine crystallizes out in beautiful white prisms, containing one-half molecule of water of crystallization, which are completely stable, in contrast to the crystals of an impure product.
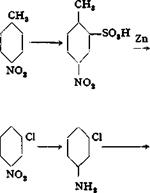
The homologous 1,2,4-toluylenediamine is prepared in the same manner as m-phenylenediamine. The procedure given above is applicable to the reduction of 2,4-dinitrochlorobenzene, 2,4-dinitreanisole, p — nitroanisole, and other insoluble nitro compounds.
m-Chloronitrobenzene from Nitrobenzene
Chlorination of nitrobenzene in the laboratory requires very careful operation and succeeds only if the reaction is carried out in the complete absence of moisture. Even traces of water prevent the chlorination or retard it excessively. It is essential, therefore, that the apparatus and the starting materials be dried thoroughly.
Anhydrous ferric chloride is the most satisfactory chlorine carrier. The commercial material is heated under reduced pressure in a round-bottomed flask through which a gentle stream of dry chlorine or hydrogen chloride is drawn. When the material begins to sublime, it is transferred to a botde with a tight glass stopper.
The nitrobenzene is dried by heating for several hours in a round-bottomed flask at 80-100°C. while a stream of dry air is drawn through it by means of a wide capillary tube.
The same apparatus recommended for the preparation of chlorobenzene (page 63, cf. Fig. 5) may be used for chlorinating a batch of 246 grams of nitrobenzene. The reflux condenser is not necessary, however, since nitrobenzene has a considerably higher boiling point than benzene. The reaction vessel should be arranged so that it can be heated easily, preferably in a water bath, and also cooled if necessary. The hydrogen chloride generated is passed through a calcium chloride drying tower and then absorbed as in the chlorobenzene preparation.
5 grams of the prepared ferric chloride suffices as the carrier. The reaction temperature should be kept between 40 and 45°C.; larger amounts of by-products are formed at higher temperatures. The rate of chlorine absorption is much lower than in the chlorination of benzene, at least 6 hours being required for the completion of the reaction. The progress of the reaction is followed by weighing the reaction flask, and the chlorination is continued until the weight has increased 85 grams. Because of the appreciable solubility of chlorine and hydrogen chloride in the reaction mixture, this weight increase corresponds to about 2 moles of chlorine actually reacted.
The chlorine stream is interrupted, and the reaction mixture is allowed to stand for a short time, then it is poured into a separatory funnel and washed thoroughly with hydrochloric acid, soda solution, and water. If part of the mixture has not already precipitated as a white crystalline solid, the mixture is transferred to a beaker until part of it solidifies, then it is filtered, cooled to about 10°, and centrifuged. About 95 grams of practically pure m-nitrochlorobenzene is obtained. The filtrate and the liquid from the centrifuge are combined and subjected to fractional distillation in vacuo. For this purpose, a column should be used which is about 80 cm. in length and provided with a partial condenser. Approximately the following fractions are collected:
(1) About 40 grams of liquid boiling at 85-106° at 9 mm. This fraction consists of nitrobenzene and some chloronitrobenzene.
(2) About 130 grams of solidifying material, boiling at 106-108° at 9 mm. This material gives on cooling and centrifuging about 100 grams of pure m-chloro — nitrobenzene. The liquid residue from the centrifuge yields only traces of chloronitrobenzene on redistillation and consists largely of a mixture of by-products (3,6-dichloronitrobenzene, p-chloronitrobenzene, o-chloromtrobenzene, etc.).
(3) Material boiling higher than 108°, and the residue. This fraction consists of the by-products mentioned. Sometimes so much hexachlorobenzene is present that it separates after long standing.
The yield of m-chloronitrobenzene is about 60 per cent, or 75 per cent allowing for the recovered nitrobenzene. The pure product boils at 107° at 9 mm. and melts at 44.5°C.
2-Chloro-4-dimethylaminobenzaldehyde
![]()
 CH,
CH,
І I
SO, H SOsNa
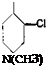 Cl
Cl
N(CH,)S
(a) p-Tolylhydroxylaminetulfonie Acid
A solution of 47.8 grams (0.2 mole) of 100 per cent sodium p-nitro. toluene-o-sulfonate (prepared from p-nitrotoluene exactly like ni-nitro — benzenesulfonic acid is made from nitrobenzene; see page 120) and 8 grams of ammonium chloride in 200 cc. hot water is exactly neutralized with ammonia. The solution is placed in an iron reduction beaker and cooled to 25°C. with stirring. The air is swept out with a stream of carbon dioxide, and then 40 grams of zinc dust is added over the course of 3 to 4 minutes through a coarse nozzle. The zinc dust must be finely distributed throughout the reaction mixture. During the reduction, the mixture must be stirred vigorously and cooled externally with ice. The reaction starts abruptly and the temperature rises rapidly as shown by the following example:
Time, min…………………….. 0 1234567 89
Temperature, °С. . . . 25 26 38 46 40 32 28 22 18 15
The mixture is filtered at once, and the zinc is washed with four 20-cc. portions of water. The filtrate and washings are combined in a graduated cylinder, the total volume measuring 240 cc.
A 2-cc. sample of the solution is taken for determining the content of p-tolylhydroxylaminesulfonic acid by means of Fehling solution. The test sample is diluted to 50 cc. and treated with 30 cc. Fehling solution [prepared by mixing equal parts of Solution I and Solution II: (I) 69.278 grams of chemically pure crystalline copper sulfate in 1 liter of solution;
(II) 346 grams of Rochelle salt and 130 grams of sodium hydroxide in water to make up 1 liter]. The mixture is heated to boiling over a hot
flame and boiled exactly 2 minutes, then cooled by immersing in cold water. The cuprous oxide is filtered off through a fritted glass filter crucible and washed with two 25-cc. portions of water. The filtrate is poured out and the suction flask is rinsed out well, and then the cuprous oxide is dissolved in an excess of sulfuric acid-ferric sulfate solution (50 grams of ferric sulfate and 200 grams of concentrated sulfuric acid dissolved in water to make 1 liter). The cuprous oxide dissolves according to the equation:
CutO + HjS04 + Fe2(S04)3 = 2CuS04 + 2FeS04 + HaO
The solution is sucked through the crucible, the latter is washed with water, and the ferrous salt determined with 0.1 N potassium permanganate solution.
The oxidation with Fehling solution does not take place stoichiome — trically. The hydroxylamine corresponding to the permanganate used is read from the curve (Fig. 22), and from this the yield is determined. Suppose, for example, that 7.9 cc. 0.1 N KMn04 were used. This corresponds to 11.1 cc. 0.1 N hydroxylaminesulfonic acid.
^9-… * = 0.133 mole hydroxylaminesulfonic acid
The yield is therefore 66.5 per cent, and 0.125 mole of p-tolylhydroxyl — aminesulfonic acid is contained in 225 cc. of the solution.
 24 сентября, 2015
24 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 