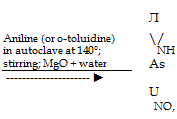
|
Cl |
Cl |
NH, |
|
|
A SO, H |
/ |
with MgO (or СаСОз) in boil |
|
|
1 —> |
+ 1 1 |
ing aqueous solution (wooden |
|
|
V |
4/ |
/ |
vat) |
|
NO, |
NO, |
NH, |
|
|
||
|
|
||
|
|||
|
|||
|
|||
|
|||
|
|
||
|
|||
|
|||
|
|||
|
|||
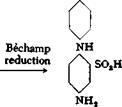
SO, H
NO, NH,
(II)
The intermediate (I), combined with aminonaphtholsulfonic acids, gives valuable dark cotton azo dyes.
 |
|
 |
|
Aminodiphenylaminesulfonic Acid (III), and Aminophenyl-
tolylaminesulfonic Acid
(III)
Aminodiphenyl-
aminesulfonic acid
These components (III) are used in preparing the nerol dyes which are disazo dyes made by diazotizing (III) and coupling with a-naphthylamine, followed
by a second diazotization and coupling with Schaeffer salt or other coupling components.
It will be recalled that not only halogen atoms, but also nitro and sulfo groups, can be replaced by phenyl — or arylamines. The lability of the nitro group, particularly in the anthraquinone nucleus, permits the preparation of some important intermediates which cannot be discussed here. Also, highly nitrated benzene derivatives give access to interesting condensation products.
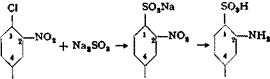
2,4- Dinitrochlorobenzene from Chlorobenzene
Cl Cl
NO,
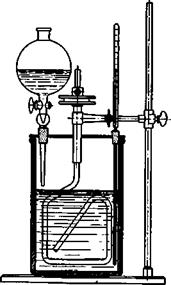 Fig. 19. Sulfonation and nitration vessel. The beaker is made of iron plate, or, better, cast iron (or porcelain or glass) and is about 11 cm. across and 20 cm. high. It is covered with a tightly fitting lead disk having openings for stirrer, thermometer, and addition tube. Stirrers of the type pictured have been found to be very practical. They can be made in the laboratory, preferably of iron, although glass rod can be used if the mechanical requirements are not too great. The bearing is best made of copper, since both iron and glass run well in copper and do not give rise toDOthersome “freezing.” The pulley is suitably made of bronze which also runs very well on copper (lubricant, petroleum jelly). The thermometer should have its scale near the top and should extend well toward the bottom of the vessel. A dropping funnel with a dropping tip should be used whenever the introduction of the liquid must be watched carefully. The vessel is placed on a rigid tripod, and the stirrer bearing is secured rigidly with a strong clamp. The thermometer and the dropping funnel should also be secured, so that the stirrer can not hit the glass tubes inside the vessel.
Fig. 19. Sulfonation and nitration vessel. The beaker is made of iron plate, or, better, cast iron (or porcelain or glass) and is about 11 cm. across and 20 cm. high. It is covered with a tightly fitting lead disk having openings for stirrer, thermometer, and addition tube. Stirrers of the type pictured have been found to be very practical. They can be made in the laboratory, preferably of iron, although glass rod can be used if the mechanical requirements are not too great. The bearing is best made of copper, since both iron and glass run well in copper and do not give rise toDOthersome “freezing.” The pulley is suitably made of bronze which also runs very well on copper (lubricant, petroleum jelly). The thermometer should have its scale near the top and should extend well toward the bottom of the vessel. A dropping funnel with a dropping tip should be used whenever the introduction of the liquid must be watched carefully. The vessel is placed on a rigid tripod, and the stirrer bearing is secured rigidly with a strong clamp. The thermometer and the dropping funnel should also be secured, so that the stirrer can not hit the glass tubes inside the vessel.
In an iron nitrating vessel (Fig. 19) is placed 280 grams of mixed acid made from 140 grams of 100 per cent sulfuric acid and 140 grams of nitric acid (sp. gr., 1.52), and to this is added, dropwise and with good stirring, 113 grams (1.0 mole) of chlorobenzene, maintaining a temperature below 5°C. Stirring is continued at 5-10° for 1 hour after the addition is
completed, then the temperature is raised slowly to 50° and held there for another hour, after which 350 grams of concentrated sulfuric acid is carefully added dropwise to the vigorously stirred mixture. The mixture is then heated at 115° for 30 minutes, cooled, and poured into 2 liters of water whereupon it soon solidifies to a light yellow cake. This is separated from the mother liquor, and melted under water to remove all the acid, yielding a product almost chemically pure. The yield is 200 grams from 113 grams of chlorobenzene. The material melts at 51°. (A warning should be given about the unpleasant properties of dinitrochloroben — zene. Especially when in solution, it causes eczema and unbearable itching.)
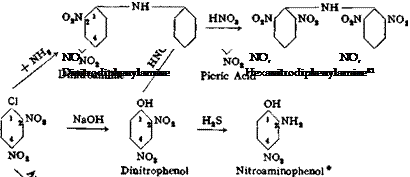 |
Technical Observations. Dinitrochlorobenzene is manufactured on a very large scale, and is used in the preparation of sulfur black T (q. v.) and other important dyes. It is, moreover, the starting material for a whole series of condensation products made by replacing the very labile chlorine atom by basic and other residues. Thus, it is easy to prepare dinitroaniline and dinitrophenol, as well as picric acid and dinitroanisole, from dinitrochlorobenzene. The accompanying formulas show only a small part of the reactions actually used (also see Table V).
We have seen how smoother nitrations are obtained in the laboratory by using a 10 per cent excess of nitric acid. In plant processes, a much smaller excess is used and even this is recovered completely by separating the waste acid into its components, sulfuric and nitric acids. This is done with steam in denitrating towers. [18]
 |
m-Phenylenediaminesulfonic Acid from 2,4-Dinitrochlorobenzene32
A solution is made of 202 grams (1.0 mole) of 2,4-dinitrochloroben — zene (page 101) in 500 cc. technical alcohol (not denatured with pyridine bases), exercising care that the solution does not come in contact with the skin (see page 102). To this solution is added the equivalent of 64 grams (1.0 mole) of S02 in the form of a saturated sodium sulfite solution. The sulfite solution is prepared from the calculated quantity of technical sodium bisulfite solution by the addition of concentrated sodium hydroxide solution. Enough sodium hydroxide is added to cause the disappearance of the yellowish color and give a faint red coloration with phenolphthalein paper. Some of the sulfite separates even from the hot solution, but this causes no difficulty. The mixture of dinitrochlorobenzene, sulfite, water, and alcohol is heated to boiling on the water bath for 5 hours with good stirring, then cooled as much as possible by placing in cold water. The sodium salt of dinitrobenzenesulfonic acid separates in lustrous yellow plates which are filtered off with suction and pressed out in a screw press. This product is reduced by exactly the same method used in the reduction of m-dinitrobenzene (see page 115). The solution of m-phenylenediaminesulfonic acid obtained in this way is not sufficiently pure for use in the preparation of azo dyes, however, and is purified by concentrating to about 400 cc. and adding 100 grams of salt. The solution is then acidified with hydrochloric acid causing the free sulfonic acid to crystallize gut. It is important to use the correct amount of acid, since the sulfonic acid will redissolve in any excess acid. Congo red paper should not turn a distinct blue, but only a weak violet. After 2 days, the precipitate is filtered off and washed with a very small amount of water. The yield is 125 grams of pure material, or about 66 per cent of the theoretical amount.
Technical Observations. Reactions of this type are carried out most satisfactorily in lead lined iron kettles, and it is important that no other metal come in contact with the liquid. A few milligrams of copper or iron prevents the formation of even traces of the desired products. The dinitrobenzenesulfonic acid, as well as the final m-phenylenediaminesulfonic acid, is not filtered in a filter press, but is
centrifuged. The residual alcohol is then removed in a hydraulic press. The alcohol is recovered by rectification and is used again. If care is exercised, the loss of alcohol in one (deration does not exceed 5 per cent.
The preceding preparation is an example of the indirect introduction of the sulfo group, utilizing the lability of the chlorine in negatively substituted aromatic chlorine compounds (see also the following preparation).
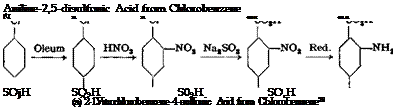
m-Phenylenediaminesulfonic acid can also be prepared easily by sulfonation of pure m-pnenylenediamine, dissolved in 100 per cent sulfuric acid, with oleum at 160°. It is used as a component in preparing azo dyes.
The reaction is carried out in a sulfonation kettle or a round — bottomed flask fitted with an efficient stirrer, thermometer, and dropping funnel, and surrounded by a larger container through which cold or hot water can be circulated (compare Figure 6, page 68, and Figure 20).
112.5 grams (1 mole) of chlorobenzene is added to a mixture of 220 grams of 100 per cent sulfuric acid and 125 grams of oleum (20 per cent S03) with stirring, over the course of about 30 minutes. The temperature rises to 70 or 80°C. When it begins to drop after the addition of the chlorobenzene has been completed, the surrounding water bath is heated to boiling and stirring is continued for about 2 hours, or until a test portion of the reaction mixture on dilution with water shows no oil droplets and has no odor of chlorobenzene when heated. (A completely clear solution is usually not obtained, because a very small amount of dichlorodiphenylsulfone is formed in the reaction. This does not separate as an oil on dilution, however, but as crystalline flocks.)
The reaction mixture is cooled in ice and 65 grams of nitric acid (sp. gr. 1.52) is added dropwise with stirring. The addition requires about 1.5 to 2 hours, and should be regulated so that the temperature of the reaction mixture remains between 15 and 20°. The cooling bath is
removed and stirring is continued for 3 to 4 hours at room temperature. The nitrochlorobenzenesulfonic acid partially crystallizes, transforming the whole mixture to a stiff paste which must be broken up with a glass rod or a spatula to permit further stirring. When a nitrometer test shows
|
Fig. 20. Autogenously welded sulfonation vessel, especially for use with oleum. |
that the residual nitric acid corresponds to the small excess used (about 2 grams), the reaction mixture is stirred with a cooled mixture of 1 liter of saturated salt solution and 500 cc. water. The precipitate is filtered off with suction and pressed out in a screw press (Fig. 21). The weight of moist filter cake is about 370 grams, consisting of about 72 per cent of solid material.
The sodium 2-nitrochlorobenzene-4-sulfonate can be obtained in a pure state by recrystallizing from hot water, dilute salt solution, or aqueous alcohol.
 20 сентября, 2015
20 сентября, 2015  Pokraskin
Pokraskin 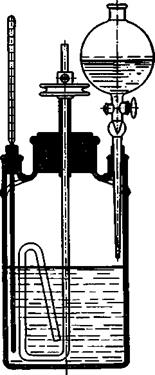
 Опубликовано в рубрике
Опубликовано в рубрике 