The Lunge nitrometer provides a simple and very useful means for controlling nitrations carried out in concentrated sulfuric acid solution. Its usefulness for the determination of the oxyacids of nitrogen, especially nitric and nitrous acids, depends on the fact that these acids (and their salts and esters) are quantitatively and rapidly reduced to NO by metallic mercury in the presence of concentrated sulfuric acid, even at ordinary temperatures, according to the reaction:
2 HNO3 + 6 Hg + 3 H2SO4——— » 2 NO + 3 Hg2S04 + 4 H20
The gaseous NO can be measured volumetrically.
Stable nitro compounds, in contrast to the nitric acid esters, are not attacked.
The nitrometer (Fig. 10) consists of a reaction and measuring tube
(a) of 50-cc. capacity graduated in 0.1-cc. divisions. At the top of this tube is a three-way stopcock connecting the tube to the filling cup (c) and the exit capillary (d). The reaction tube is connected at the bottom with the leveling tube (b) by means of thick-walled rubber tubing.
For carrying out a determination, 1 to 2 grams (about 1 cc.) of the nitration mixture is weighed out in a small test tube to an accuracy of 0.01 gram. After the measuring tube (a) has been filled up to the stopcock with mercury, the sample is transferred to the filling cup (c) and then run into the measuring tube by lowering the leveling tube
(b) and carefully opening the stopcock. Care must be taken that no air is admitted to the measuring tube. The weighing tube is rinsed three or four times with 1-2 cc. portions of concentrated sulfuric acid, which
are transferred successively into the measuring tube in the same manner, in order to get all traces of the sample into the tube. The total volume of the acid layer over the mercury should be about 6-10 cc. The measuring tube is now removed from its clamp and, while the stopcock is carefully held closed with one hand, brought to a nearly horizontal position so that the acid layer moves toward the other end of the tube. Before the acid reaches the rubber tube connection, the
|
|
|
Fig. 10. The Lunge nitrometer. |
tube is quickly righted while being shaken so that the acid passes through the mercury up the tube. This operation of inverting and righting the tube is continued for about 5 minutes in a manner to bring about the most intimate contact between the acid and the mercury. If the original sample contained nitric acid, gas bubbles soon begin to form and collect over the acid. When the volume of gas no longer increases, the measuring tube is replaced in the clamp, and the leveling tube is positioned so that the top of the mercury column in it stands higher than that in the measuring tube by about one-seventh or one — eighth of the height of the acid layer (ratio of sp. gr. of mercury to sul-
furic acid = 13.6 : 1.84 — 7.4). The volume of gas over the acid is read, then the shaking operation is repeated for a few minutes and the volume is read again to ensure that the gas volume is no longer increasing.
If a relatively large amount of nitric acid is present in the nitrating mixture being tested, the gas formation starts to take place very soon after shaking is begun, and the gas bubbles assist greatly in mixing the mercury and acid layers, so that the reaction is soon completed. If, on the other hand, only a little nitric acid is present, a considerable time may elapse before the first gas bubbles are formed. In these cases, shaking must be continued uninterruptedly for at least 5 minutes before all the nitric acid is decomposed.
The amount of nitric acid in the sample of nitrating mixture is calculated from the volume of NO in the following manner: If an exact determination is desired, the temperature and barometric pressure must be taken at the time the volume of NO is read, and the gas volume corrected to 0° and 760 mm. pressure; this volume is multiplied by 1.3402 (weight in milligrams of 1 cc. NO at 0° and 760 mm.) to obtain the weight of NO in milligrams. Such high precision is not necessary in controlling nitrations; hence the corrections can be dispensed with, and the weight of NO calculated on the basis of an average laboratory temperature of 20° and average barometric pressure, according to the values given in the following table:
Barometric pressure, mm. . . 700 710 720 730 740 750 760 770
Mg./cc. ЫсГаГгЬ0 …. 1.15 1.17 1.18 1.20 1.22 1.23 1.25 1.26
Mg. HNO3 corresponding
to 1 cc. NO…………………………. 2.41 2.45 2.48 2.52 255 2.59 2.62 2.66
The weight of NO is multiplied by 2.1 to give the weight of HNO;i,
since HNO^ : NO = 63 : 30 = 2.1. The bottom line in the table gives the weight of HNO;f, thus calculated, corresponding to 1 cc. NO at the various pressures. Multiplication of this weight of HNO>( per cubic centimeter NO by the volume of NO gives the weight of HN03 in the weighed out sample. One has only to multiply this latter weight by the total weight of the nitration mixture and divide by the weight of the sample to get the total weight of HNO;!, in milligrams, in the nitration mixture.
The nitrometer test is usable only for nitrations which are carried out in concentrated sulfuric acid solution. It is to be noted that nitrous acid and nitrosylsulfuric acid also generate NO in the nitrometer. If, as is often the case, nitration is accompanied by some oxidation, the HN02 formed is determined along with the HNO3. On the other hand, this reaction of nitrous acid makes it possible to use the nitrometer in controlling diazotization and nitrosation reactions when these are run in concentrated sulfuric acid solution.
|
3. Aniline from Nitrobenzene
|
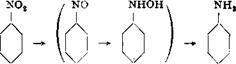
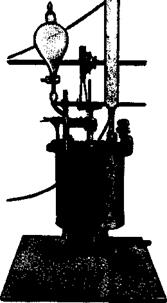
Reduction of nitrobenzene to aniline is carried out in a copper or iron vessel which is tightly covered and equipped with a stirrer reaching to the bottom of the vessel. In the laboratory, a kettle such as the one shown in Figure 11 is commonly used. In the apparatus, fitted with reflux condenser and dropping funnel, are placed 200 grams of cast iron shavings ground to a fine powder, 300 cc. water, and 20 cc. 30 per cent hydrochloric acid. The mixture is boiled for 10 minutes to etch the iron, and then, with continuous stirring (the iron must be thoroughly stirred up), 123 grams (1.0 mole) of nitrobenzene is added over a period of 45 minutes. The mixture is kept at the boiling point throughout, but since the reduction generates considerable heat, the external heating can be diminished or entirely removed. The iron is almost completely oxidized to Fe304, which deposits as a thick, easily filtered precipitate. Boiling is continued until the liquid running back from the reflux condenser is completely colorless. Then 15 grams of soda ash is carefully added to the reaction mixture (foaming!), and the aniline is distilled out with steam. Steam is introduced through one of the side openings, the condenser is connected to the main opening by means of a bent glass tube which should be at least as wide as the steam tube, and the third opening is plugged.
Aniline is somewhat soluble in water (3.0 grams in 100 grams of water); therefore enough salt should be added to the distillate to make it a 20 per cent salt solution in which aniline is very insoluble. After standing for several hours, the aniline can be separated in a separatory funnel and distilled over a free flame (see Fig. 7, page 71). The first fraction, containing traces of benzene and some water, is removed, and the main fraction is collected, about 99 per cent boiling at 182°C. The yield of aniline from 123 grams of nitrobenzene is about 85 grams, or 91 per cent of the theoretical amount.
Technical Observations. In large scale operations, the aniline is distilled with steam which is already saturated with aniline. This is done by feeding the steam kettle with the waste water from the steam distillation process. In the Weiler-Ter
Meer process, the aniline water is simply extracted with nitrobenzene which removes the aniline completely, and then the aninne-nitrobenzene solution is reduced directly. This process avoids the disadvantages attending the use of aniline water in the steam kettle.
Tn the industrial preparation of aniline, the iron is added gradually and less water is used.[13] The yields obtained are practically quantitative; about 110 kilograms of pure aniline from 100 kilograms of benzene. The aniline is vacuum distilled in batches of 10,000 to 30,000 kilograms, the heat being supplied by a system of steam cods inside the kettle.
In recent times, the reduction of nitrobenzene to aniline by hydrogen or water gas, in the presence of suitable catalysts, has been introduced. Compared to the iron reduction method, the catalytic method has the advantage of being a continuous process and therefore requiring a considerably smaller apparatus for the same production.[14]
It should be mentioned incidentally that alkali metal sulfides can also be used as reducing agents for this purpose if they happen to be available cheaply.
An important new process for the production of aniline, originating with Dow Chemical Company, has attained great significance. Dow Chemical Company produces large quantities of aniline (also phenol by an analogous method, see page 88) from chlorobenzene and ammonia. The reaction is carried out at very high pressure (up to 340 atmospheres) and temperatures (340°), forming aniline and ammonium chloride.
The coal tar dye industry received its first stimulus when the manufacture of aniline was started — first in England — and aniline has remained one of its most important products. A considerable amount of aniline is still used today in the production of aniline black on the fiber. Large quantities are also used in the preparation of dyes of various classes, either directly or after conversion into numerous other intermediates. Aniline is also an important ‘starting material in the preparation of pharmaceutical products. Apparently, the largest consumer of aniline derivatives in modern times, however, is the rubber industry, which uses huge quantities of diphenylguanidine, thiocarbanilide, and other sulfur containing aniline derivatives as vulcanization accelerators.
Aniline and nitrobenzene, and many of their homologs and substitution products, are strongly poisonous. They produce the so-called “aniline poisoning” which is a blood poisoning accompanied by cyanosis (i. e., blue coloration of the skin, especially noticeable around the lips and finger nails, due to an excess of CO2 in the blood). These compounds are especially dangerous because they can be absorbed not only through the stomach, but also through the respiratory system, and even through the skin. In all plants where these products are made or worked with, therefore, good ventilation and efficient removal of dust and vapor should be provided, and the greatest cleanliness should be practiced (washing of hands before meals, bathing and laundering of clothing after each work day). More malignant than the acute poisoning and cyanosis is cancer of the bladder (more recent experience indicates that /5-naphthylamine is chiefly responsible for the origin of cancer of the bladder) which often occurs after many years of working in an aniline plant and is not generally recognized until it is too far advanced to be remedied. In addition to the precautionary measures already mentioned, the workers in aniline plants should be under constant medical supervision and should be transferred to nonhazardous work at the first sign of impairment of health.
й
|
Fig. 11. Closed, coppered reduction vessel fitted with stirrer, dropping funnel, and reflux condenser, for use with substances volatile with steam. |
 17 сентября, 2015
17 сентября, 2015  Pokraskin
Pokraskin 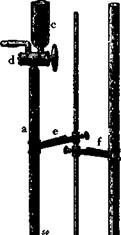

 Опубликовано в рубрике
Опубликовано в рубрике 