Nitration of aromatic compounds is carried out industrially, in most cases, using a mixture of nitric and sulfuric acids (mixed acid or nitrating acid). The sulfuric acid serves to take up both the water formed in the nitration reaction and
that originally present in the nitric acid; in this way a sufficiently high concentration of nitric acid is maintained throughout the reaction, and almost complete utilization of the nitric acid is made possible. Sulfuric acid is also an excellent solvent for many substances. Due to its high heat capacity, the sulfuric acid serves to absorb die heat of reaction, making for a smooth and uniform reaction. When used in large excess, sulfuric acid even protects a free primary amino group from attack by nitric acid (cf. 2-nitro-4-aminotoluene, page 165).
In dye plants, a whole series of nitrating acids is kept on hand, prepared by mixing, with cooling, nitric acid of various concentratiens with concentrated sulfuric acid or with oleum. From these various mixtures, containing different amounts of water and different ratios of nitric to sulfuric acids, one can be selected as suitable for any specific nitration. The more difficult a substance is to nitrate, the less water should be present in the mixed acid. Indeed, in many cases a mixed acid containing free SO3 is used (see page 150).
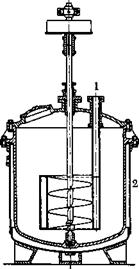
Since nitration is a strongly exothermic reaction, good cooling should be provided, as a rule, particularly since many nitrations go smoothly, only at lower temperatures; at higher temperatures, oxidation or some other side reaction may be strongly favored. If several isomers are formed simultaneously in a nitration, the temperature may have some, but usually a rather small, influence on the relative amounts of the isomers formed. In order to avoid a dangerous rise in temperature on the addition of the nitric or mixed acid, it is absolutely necessary to have continuous, vigorous stirring in all nitrations, especially if the substance being nitrated does not dissolve in the nitric acid, as in the case of benzene. Under the latter condition, two layers are formed if no stirring is used, and when the reaction starts at the liquid-liquid interface, strong local overheating occurs which might lead to an explosion. A terrible explosion once occurred during the preparation of nitrobenzene in the aniline plant at Rummelsburg, when the nitrating acid was added while the stirrer was not running and the stirrer was started later. Since then, such accidents have been prevented by the use of devices which permit the addition of the acid only when the stirrer and the cooling system are functioning. Accidents have happened in the laboratory when the stirrer was installed so that it did not reach to tne liquid level at the start of the reaction; in a matter of seconds after the liquid level reached the stirrer, an explosive reaction occurred. Extreme precautions are taken in the laboratory, therefore, especially when using nontransparent reaction vessels, to see that the stirrer and the thermometer are immersed in the liquid at the start.
The type of procedure described in the foregoing example — addition of the mixed acid to the substance being nitrated — is used only in a few, but technically very important, nitrations. More often, the substance to be nitrated is dissolved in concentrated sulfuric acid and to this solution is added nitric acid or mixed acid with stirring and cooling. The use of mixed acid here has the advantage that the heat arising from mixing nitric and sulfuric acid is not generated in the reaction mixture, and thus does not add to the heat of nitration. If a nitration follows a sulfonation reaction, it is usually carried out right in the sulfonation mixture (cf.
2- nitrochlorobenzene-4-sulfonic acid, page 104).
In many nitrations, for example, of primary amines, the nitric acid must be completely free from nitrous acid; in such cases, before the nitric acid is used, or before it is mixed with the sulfuric acid, it should be freed from nitric oxide by blowing air through it (cf. nitration of p-toluidine, page 165). It is also possible to add saltpeter, instead of nitric acid, to the sulfuric acid solution; this procedure is less satisfactory, however, because saltpeter is not readily soluble in sulfuric acid at low temperatures, and a steady reaction is hard to maintain.
It is often observed that prolonged treatment with the nitrating mixture results in a lowering of the yield and purity of the product; it is advisable, therefore, to remove the product immediately after the nitration is complete. Using the
|
|
|
|
Lunge nitrometer, the consumption of the nitric acid can easily be followed and the end of the reaction determined.
The presence of concentrated sulfuric acid, in many cases, influences the position taken by the nitro group. For example, it weakens the orienting action of acetylated or formylated amino groups, and that of free amino groups to an even greater extent; the other substituents present may then have an influence on, or even determine, the position taken by the nitro group. This effect may be undesirable and make it necessary to avoid the use of mixed acid.
In addition, substances which are easily sulfonated, hydrolyzed, or otherwise changed, by concentrated sulfuric acid cannot be nitrated in sulfuric acid solution. In these circumstances it is necessary either to use aqueous nitric acid of suitable concentration, or to carry out the nitration with concentrated nitric acid in a solution or suspension in an organic liquid. The organic liquid must be one which is affected as little as possible by nitric acid under the conditions prevailing in the process (for example, glacial acetic acid, nitrobenzene, or o-dichlorobenzene).
Indirect introduction of a nitro group into an aromatic nucleus is rarely encountered in industrial operations.
 16 сентября, 2015
16 сентября, 2015  Pokraskin
Pokraskin 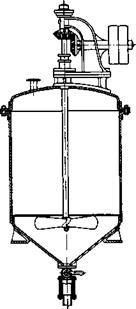
 Опубликовано в рубрике
Опубликовано в рубрике 