Chlorination is almost always carried out technically by the direct action of gaseous chlorine. Substances which are liquid at the reaction temperature are usually treated, without dilution, with chlorine gas, as described above for
benzene. Solid substances, on the other hand, must usually be dissolved or suspended in a suitable liquid. For this purpose, of course, only those solvents are suitable which are attacked with difficulty, or not at all, by chlorine under the conditions employed. Suitable inorganic liquids are water and concentrated sulfuric acid, and the organic liquids most commonly employed are carbon tetrachloride, tetrachloroethane, nitrobenzene, o-dichlorobenzene, trichlorobenzene, and glacial acetic acid. Efficient stirring should be provided in all cases in order to ensure good utilization and uniform action of the chlorine. It is advantageous to introduce the chlorine in a finely divided state, for example, by passing it through a cylinder of porous material (porous stone). It is also recommended, particularly if the chlorine is taken up slowly, that a high and relatively narrow vessel be used so that the gas is made to pass through as thick a liquid layer as possible.
Many chlorinations take place smoothly only if no trace of water is present. In these cases, the substance to be chlorinated, the solvent, and the chlorine should all be dried as carefully as possible. Even in those cases where the chlorine might be used without drying, it should be passed through a wash bottle containing sulfuric acid so that the velocity of the gas stream can be observed. The progress of the chlorination is followed by weighing the chlorine cylinder, the reaction flask, or the flask for absorbing the hydrogen chloride, the latter only if no unused chlorine is collected. In this connection, it should be noted that only one-half of the chlorine is used in the substitution reaction, the other half escaping in the form of hydrogen chloride. It should also be remembered that not only hydrogen chloride, but also chlorine, may be appreciably soluble in the reaction mixture, and for this reason a rapid absorption of chlorine may be deceiving. In addition, the stream of escaping hydrogen chloride gas can easily entrain volatile substances or solvents. Their loss is prevented by using an efficient reflux condenser.
Because of the high toxicity of chlorine, the whole chlorination apparatus should be placed under an efficient hood and tested carefully for leaks. Long lengths of rubber tubing should not be used, because rubber is rapidly destroyed by chlorine. All joints through rubber tubing should be wired.
The optimum conditions for chlorinations vary within wide limits, depending on the nature of the starting material. Aromatic hydrocarbons, as well as their halogen and nitro derivatives, usually undergo nuclear chlorine substitution only in the presence of a halogen carrier. In the absence of a carrier, the chlorine adds, or a side chain may be chlorinated if one is present. In industrial operations, the most commonly used carrier is iron,.either as the metal or in the form of ferric chloride. Sometimes iron is used in combination with iodine, and less often, iodine itself, antimony, or antimony chloride is used. In order to bring about the substitution of chlorine in the side chain of a homologous aromatic hydrocarbon, it is necessary to exclude, very carefully, the aforesaid carriers and to work at higher temperatures, usually under illumination; it is also often advantageous to add phosphorous or its chloride.
Amines are seldom chlorinated in the free form because of the strong oxidizing action of chlorine. An exception is p-nitroaniline which reacts smoothly with chlorine in the presence of concentrated hydrochloric acid to yield, first, 2-chloro 4-nitroaniline, and then, 2,6-dichIoro-4-nitroaniline:
|
Usually, it is necessary to protect the amino group by acylation and carry out the chlorination under cooling, in order to avoid side reactions. These amino dervatives react very readily with chlorine even in the absence of a carrier.
Halogenation of phenols takes place still more easily, so that with these it is often difficult to prepare a monohalogen derivative by direct action of free halogen. In order to obtain monochlorinated phenols, it is usually necessary to use a mild chlorinating agent. Sodium hypochlorite is used for this purpose. It reacts smoothly with phenol to yield the monochloro compound, predominantly o-chlorophenol, when used in equimolecular quantity in alkaline solution. Sulfuryl chloride (SO2CI2), on the other hand, reacts with phenol to yield chiefly the p-chlorophenol (see page 145). Sulfuryl chloride is usable not only for phenols, but finds frequent use as an easily controllable chlorinating agent, especially in the anthraquinone series.
Sometimes it is possible to obtain an energetic and smooth chlorination by the use of nascent chlorine, generated in the reaction mixture itself, from hydrochloric acid and an oxidizing agent. Suitable oxidizing agents are hypochlorite (e. g., in the chlorination of acet-o-toluidide), chlorate, or even nitric acid (e. g., in the preparation of. chloranil by means of aqua regia, see page 146).
Under some conditions, chlorine can replace not only hydrogen, but also certain substituent groups such as sulfo, carboxyl, nitro, etc. Use is made of these reactions especially in the anthraquinone series (see page 236). They are, however, not unknown in the benzene series. Thus, p- toluenesulfonyl chloride, when treated with chlorine at a high temperature in the absence of iron and antimony but in the presence of phosphorus pentachloride, gives p-chlorobenzo — trichloride (replacement of the —SO2CI group by chlorine):[11]
What has been said about chlorination applies generally for bromination, except that bromine is usually added in the liquid form to the reaction mixture. In isolated cases, however, better results are obtained if the bromine is introduced in the gaseous form. With sensitive substances, bromination often takes place more smoothly than chlorination and yields a purer, more easily crystallized product. Under these circumstances, the higher price of bromine is more than offset by the better yields. If concentrated sulfuric acid is used as the solvent in brominations, the hydrogen bromide formed in the reaction is more or less completely oxidized back to bromine by the sulfuric acid. In such cases, less than the calculated amount of bromine is used, sometimes only one-half or a little more.
Iodination and fluorination are encountered only rarely in dye chemistry.
It is not always possible to introduce, by direct halogenation, a chlorine or a bromine atom entirely or predominantly into the desired position. Thus, m-dichloro — benzene cannot be obtained by further chlorination of chlorobenzene. Similarly, o-chlorotoluene cannot be prepared satisfactorily by direct chlorination of toluene, because it is always formed along with the para compound and the two cannot be separated in any practical way. For these preparations, it is usually necessary to use the Sandmeyer reaction (replacement of an amino group by halogen through the diazonium compound), although it is a rather costly and unreliable technical process and seldom runs smoothly. In other cases, a roundabout method can be used, involving blocking the position where the halogen is not desired by means
of a sulfo group, and then splitting out the sulfo group after the halogenation reaction.[12]
NO,
 |
 |
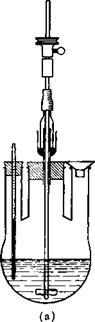
In a three-necked flask fitted with an efficient stirrer, thermometer, and dropping funnel (see Fig. 6) 100 grams of benzene is placed. To
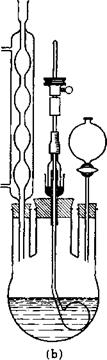
Fig. 6. (a) Three-necked flask fitted with thermometer, filling funnel, and propeller stirrer with gas-tight seal; (b) three-necked flask fitted with reflux condenser, dropping funnel, and paddle stirrer with gas-tight seal.
this is added, over a period of about 30 minutes, with vigorous stirring, a cooled mixture of 110 grams of nitric acid (sp. gr. 1.44, 44° Be, 75 per cent HN03) and 170 grams of concentrated sulfuric acid (66° B6). The temperature of the reaction mixture is held at 50°C. by external cooling. Instead of a glass flask, a porcelain beaker having a tight cover, or a covered enameled kettle can be used. In any case, it is essential that both the thermometer and the stirrer are immersed in the liquid at all times. In order to ensure a smooth nitration, nitric acid of sp. gr. 1.46 (46° Be, 80 per cent HN03) may be used; in the plant, the 44° Вё acid gives good results. When the addition of the mixed acid is completed, the mixture is stirred for 2 hours at 50°, and then the temperature is raised to 60°. Completion of nitration is established by a nitrometer test showing that the residual nitric acid corresponds to the small excess used. The nitrobenzene is separated from the acid layer in a separatory funnel, washed with water, then with soda solution, .and finally with water. The neutral nitrobenzene (litmus test) is distilled directly, giving a first fraction containing water and a small amount of benzene, and then a fraction of pure nitrobenzene. High boiling liquids are usually distilled in the simple apparatus shown in Figure 7; sometimes the Liebig condenser is omitted and the receiver is cooled by a stream of water.
The yield of pure nitrobenzene boiling at 205° is about 150 grams, or 95 per cent of the theoretical amount.
Technical Observations. Nitrobenzene is one of the large volume products of tlie dye industry. It is used in the preparation of aniline and benzidine, as well as a series of other intermediates, particularly those of the meta series (see Table VI); it is also used in the preparation of the important dye, nigrosine. Nitrobenzene is also used as a solvent, and occasionally as a mild oxidizing agent (fuchsin fusion, quinoline synthesis). In the industrial preparation of nitrobenzene, batches of up to 1500 kilograms of benzene are used and yields of 98 per cent are obtained. A large scale nitration requires about 12 hours, and up to 97 per cent of the nitric acid is utilized. The course of nitration is followed by quantitative determination of the nitric acid in the reaction mixture, using the Lunge nitrometer. At the end of the reaction, the waste acid should contain only about 1 per cent of nitric acid. The nitrobenzene is commonly used without further purification, but if it is to be purified, vacuum distillation is always used.
Figures 8 and 9 show a nitration kettle with inner cooling, such as is used for nitrating aromatic hydrocarbons, and a separating funnel equipped with a hard lead or ceramic stopcock and having a window (lunette). The apparatus used for nitrating benzene must be completely lead lined because the spent acid is highly diluted at the end of the nitration and attacks iron.
 15 сентября, 2015
15 сентября, 2015  Pokraskin
Pokraskin 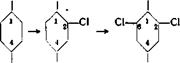
 Опубликовано в рубрике
Опубликовано в рубрике 